|
|
Acid - donates a proton |  |
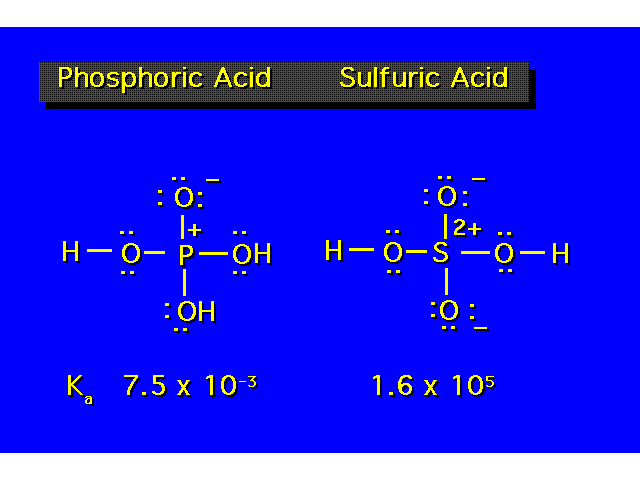
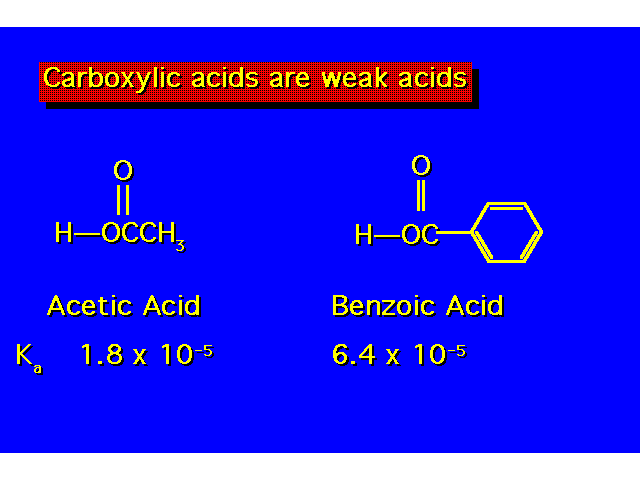
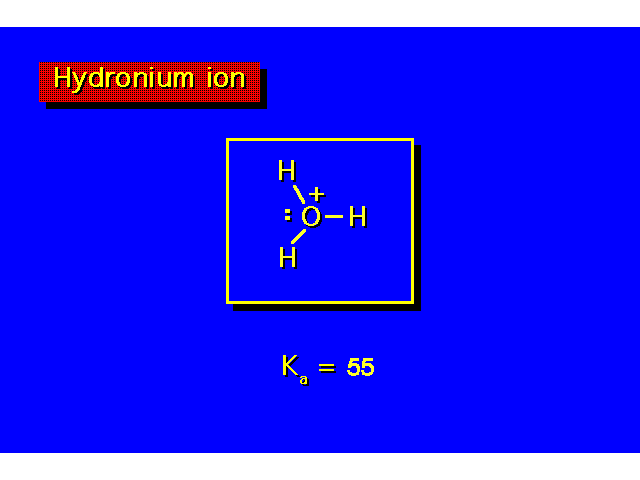
| The value of Ka indicates the strength of the acid. The larger the value of Ka, the greater the ionization & the stronger the acid. The smaller the value of pKa the stronger the acid. | 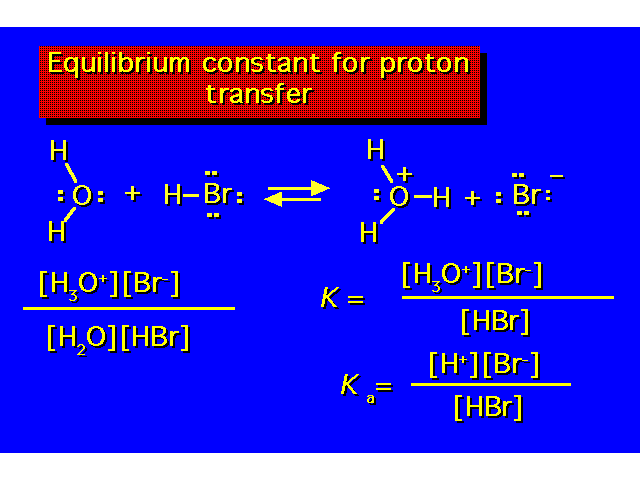 |
| Acid strength increases as you go down a family even though this is opposite the electronegativity trend. The increase in acidity down the family parallels the decrease in bond strength. As the halogen becomes larger, its orbitals overlap less efficiently with the hydrogen 1s orbital. | 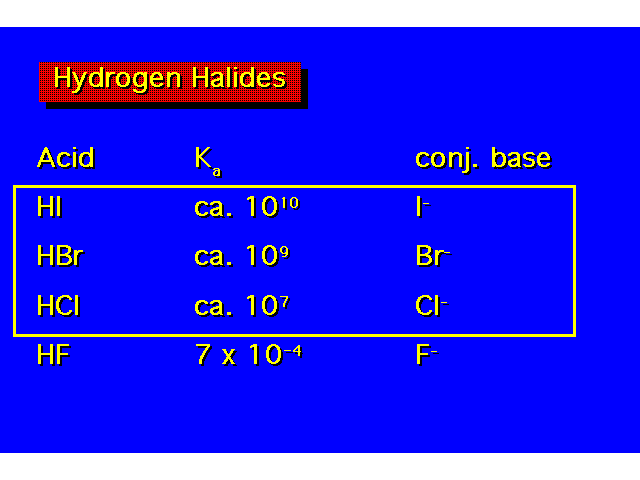 |
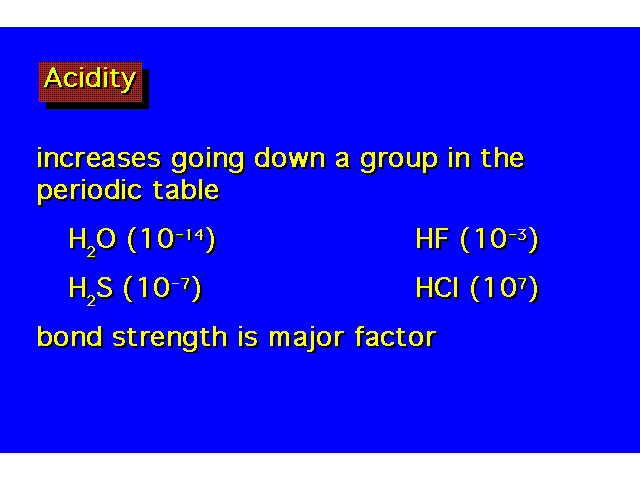 | |
 | |
 | |
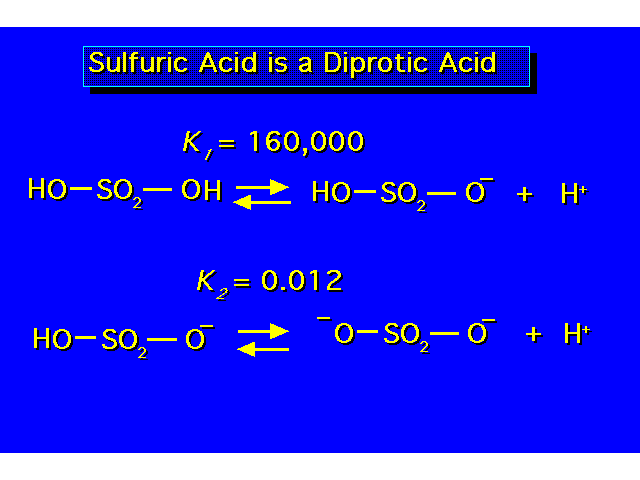 | |
 | |
 | |
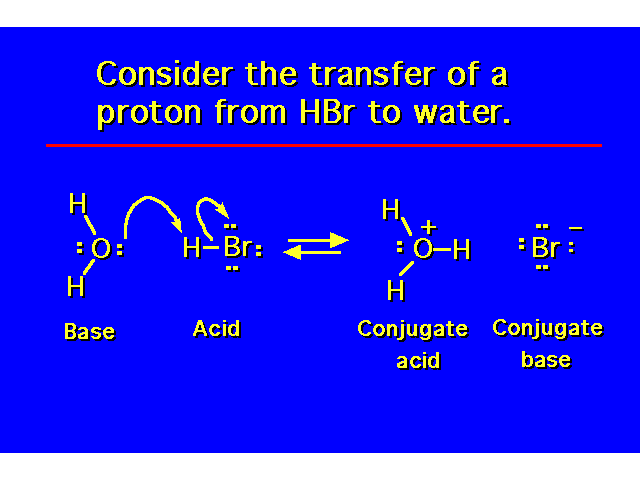
| In organic chemistry, there are certain conventions that must be obeyed in showing electron movement in reactions. Arrows are always drawn showing where the electrons start with the tail of the arrow and where they will end up with the head of the arrow. | 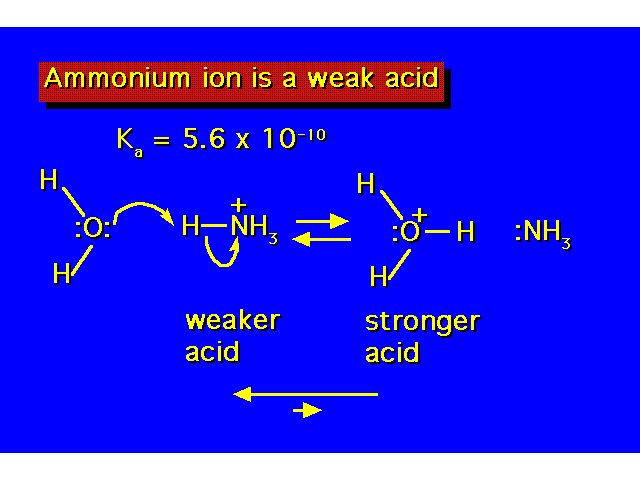 |
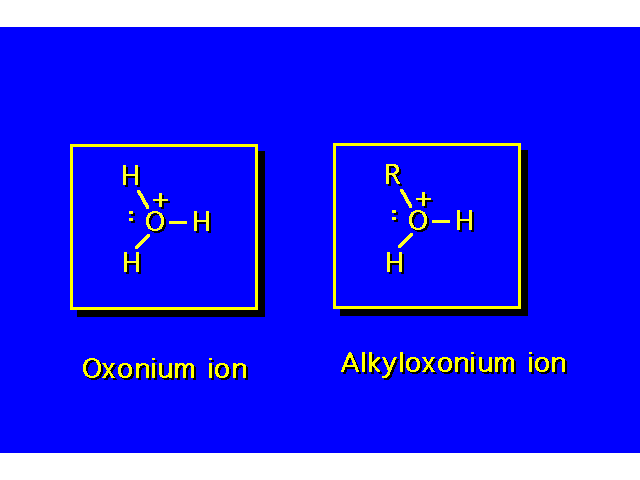
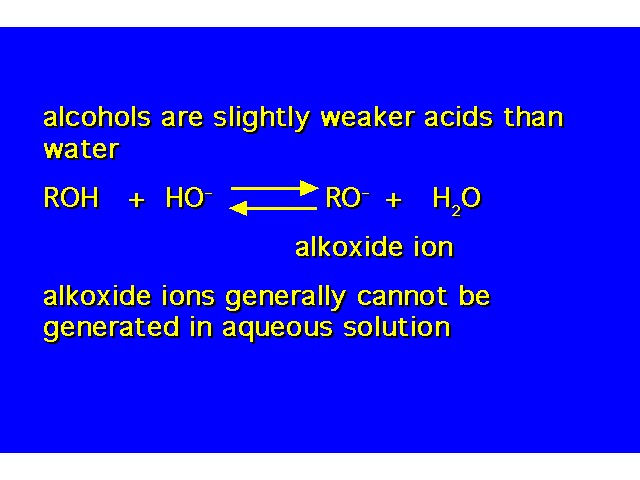
| The alcohol is both a Bronsted-Lowry base and a Lewis base. Lewis bases are electron donors. Lewis acids are electron acceptors. |  |
 | |
 | |
| POTENTIAL ENERGY DIAGRAMS Potential energy diagrams show how energy changes during the course of a chemical reaction. This is a diagram for an exothermic process (reactants higher in energy than products so energy released to the environment). All reactions possess an energy barrier that must be overcome to go from reactants to products called the activation energy. The transition state (TS) is the configuration of the atoms at the energy maximum in the potential energy diagram. Unlike reactants or products, a TS is unstable and cannot be isolated. An intermediate is a species that exists for some finite length of time. The intermediate is actually a product in one step of the reaction sequence, and the reactant in the next step. | 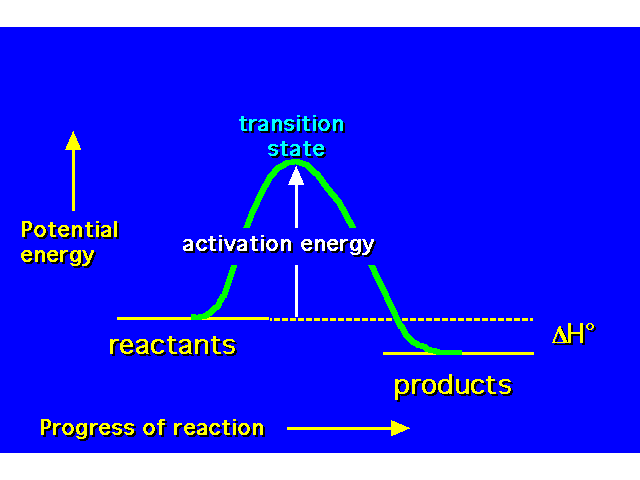 |
| This is an example of a concerted process -- a chemical transformation involving only one step. All bond making and bond breaking occurs simulaneously. This potential energy diagram shows how the energy changes as the bond between the water and the hydrogen forms at the same time the bond between the hydrogen and the bromine breaks. | 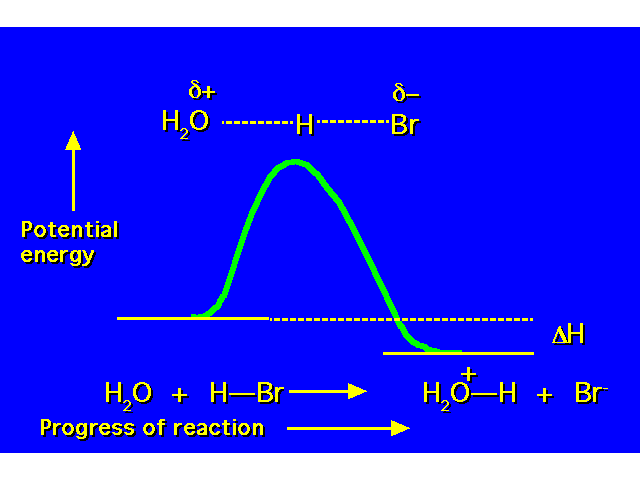 |
 | |
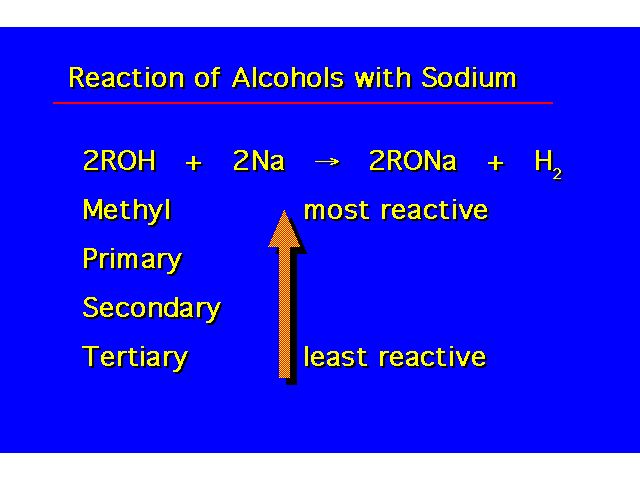
| This reaction is irreversible. The hydrogen gas escapes from the reaction mixture shifting the equilibrium to the right. This reaction must be done in the absence of water -- water would react violently with the sodium. Typically, an excess of alcohold is used to serve as the solvent. |  |
 | |
