Molecular Fingerprints
| What is Molecular Fingerprinting? |
Important Equations/Constants/Variables: |
||
| As described in the last section, when bombarded with light energy in the form of photons, each and every molecule will have ground state electrons that will absorb that energy and be moved to a higher energy level; and then it will decay from a higher energy level to a lower energy orbit (back to its ground state) by giving off a photon (i.e. light energy). As it turns out, every single molecule absorbs and emits light at specific, and unique, wavelengths. Because of this fact we are able to observe the light emission spectrum of an unknown molecule and compare those wavelength peaks to the peaks of known spectra; and by finding what peak, or set of peaks, the unknown molecule's spectra corresponds to we are able to know the identity of the unknown molecule. Below is raw data (in graphical form) for two molecules (sodium and hydrogen) that I measured experimentally. I then compared them to the known spectra of both molecules. The results are discussed below. Click on the following link to learn about some of the potential applications of molecular fingerprinting through emission spectra. |
Constants: c = 3*108 m/s Speed of Light h = 6.636*10-34 J*s Plank's Constant d = 1666 nm Variables: λ = wavelength (in meters) f = frequency (in hertz or s-1) E = energy transfer (in J) Equations: f = c/λ Equation I f = E/h Equation II E = (c*h)/λ Equation III d*sinθ = m*λ (m = 0, 1, 2, ...) Equation IV |
||
| Raw Spectrum Data: | |||
|
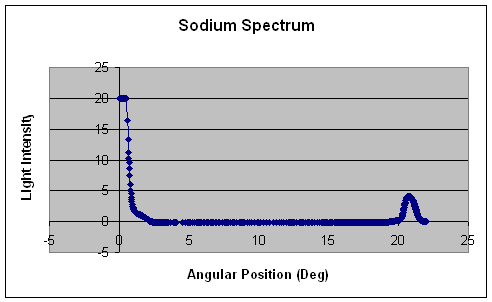
Right: To the right is the raw data for the sodium spectrum. The graph is depicted as angular position (in degrees) versus light intensity. By setting the angular position equal to θ and plugging it into equation IV, you are able to calculate the wavelength. Here in this case we observe a single peak at about 20.7o which correlates to a wavelength of 589 nm. At closer observation, the peak is a little broad and is not symmetrical. This indicates that there may be a double peak in this region. By comparing my experimental value for the single sodium peak with the known sodium spectrum, I found that you should expect to find two peaks at 589 nm and 589.6 nm which would explain the broad, nonsymmetrical peak. |
 |
||
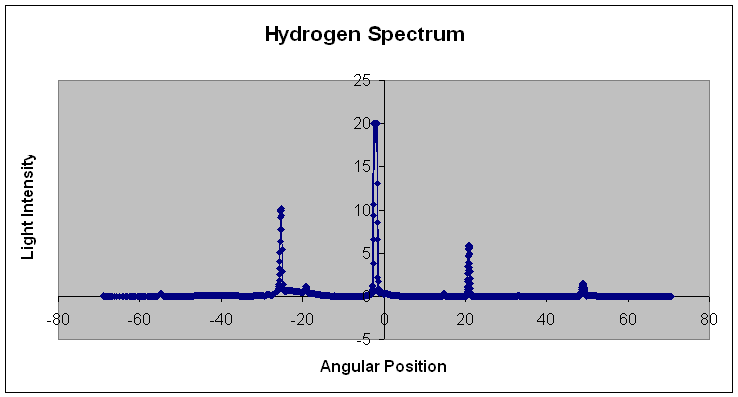
| Below: Below is the raw experimental data for the hydrogen spectrum. To determine the wavelengths of each of the peaks, the same calculations for the sodium spectrum were used. Peaks were found at 14.25o, 15.1o, 17.0o, and 23.2o which correspond to wavelength values of 410 nm, 434 nm, 487 nm, and 656 nm. These experimental values for the hydrogen spectrum match up perfectly with the known values for the hydrogen spectrum. | |||
 |
Youtube.com provides an excellent movie clip on molecular fingerprinting: |