Ionic Bonding | 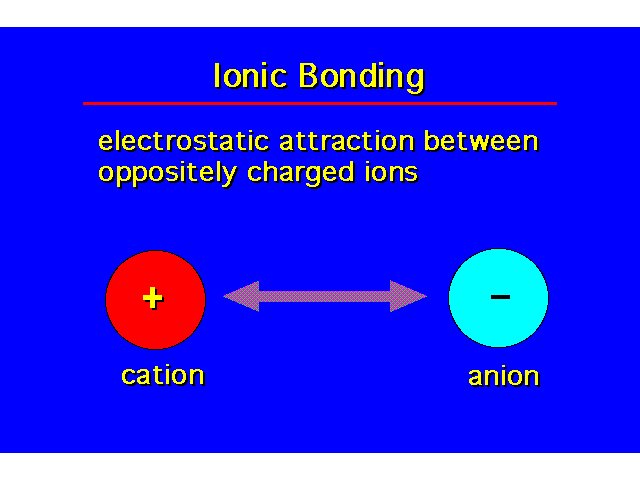 | ||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
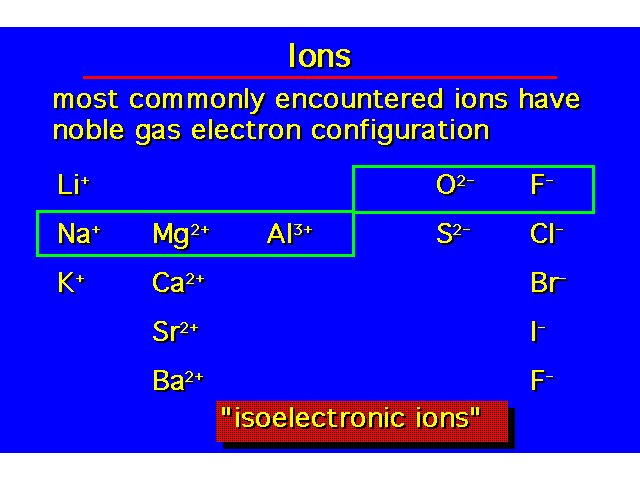 | |||||||||||||||||||||||||||||
Covalent Bonding | 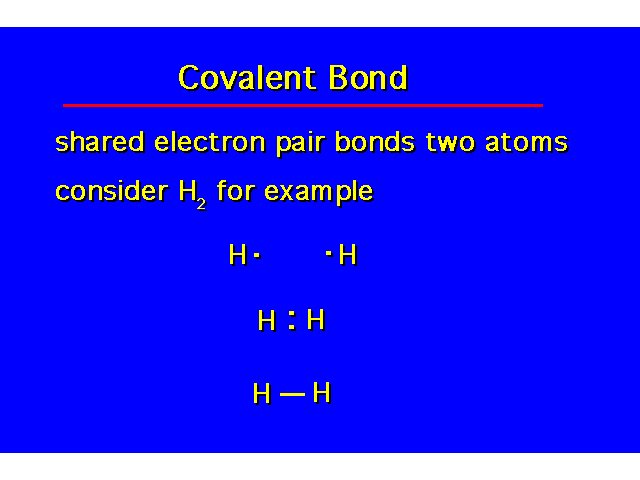 | ||||||||||||||||||||||||||||
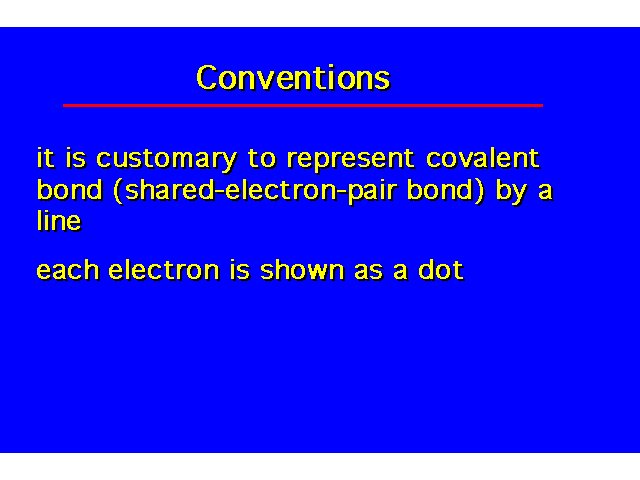 | |||||||||||||||||||||||||||||
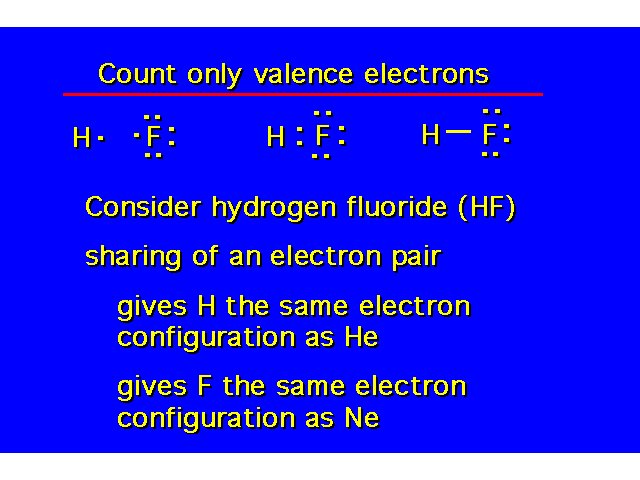 | |||||||||||||||||||||||||||||
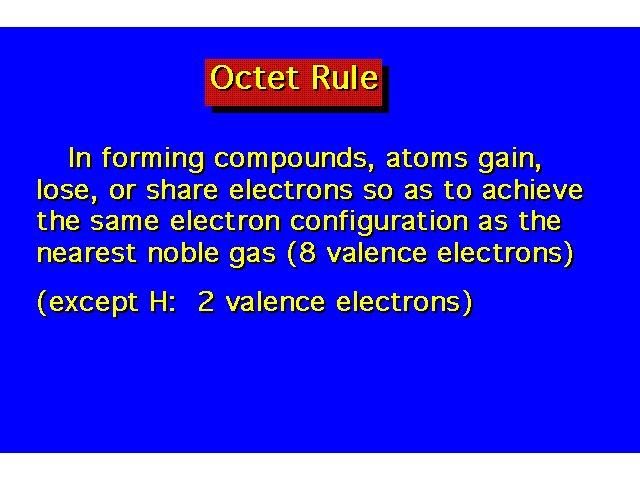 | |||||||||||||||||||||||||||||
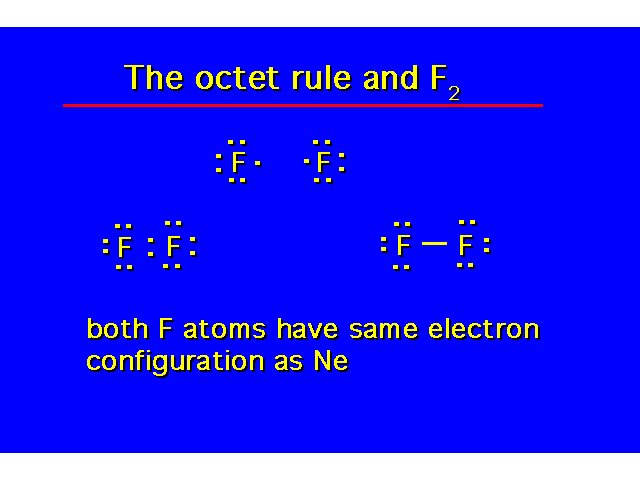 | |||||||||||||||||||||||||||||
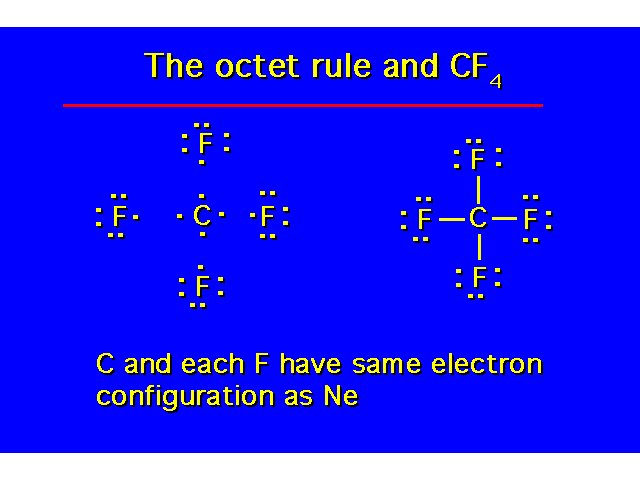 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
| Another aid: S = N - A S = total # e-shared N = total # e- needed to complete octets A = total # e- available to molecule (sum of valence e- adjusted for any ionic charges |  | ||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
| S = N - A S = [1(2) + 2(8)] - [1 + 6 + 7] S = [18 - 14] S = 4 shared e- or 2 covalent bonds |  | ||||||||||||||||||||||||||||
| S = N - A S = [3(2) + 2(8)] - [3(1) + 5 + 6] S = [22 - 14] S = 8 shared e- or 4 covalent bonds |  | ||||||||||||||||||||||||||||
Multiple Bonds |  | ||||||||||||||||||||||||||||
| S = N - A S = [1(2) + 3(8)] - [1 + 2(6) + 5] S = [26 - 18] S = 8 shared e- or 4 covalent bonds |  | ||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
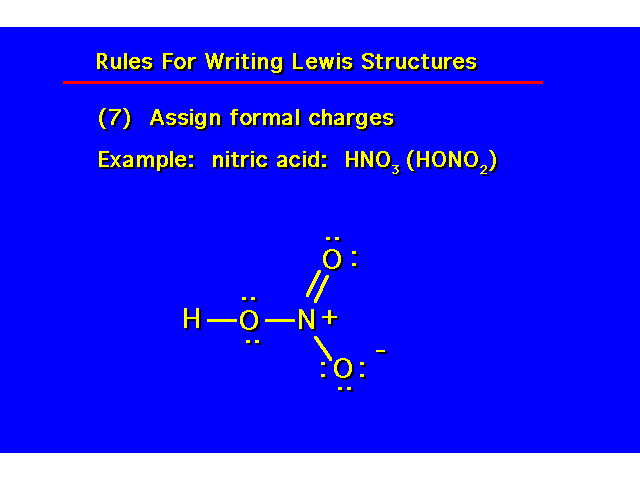 | |||||||||||||||||||||||||||||
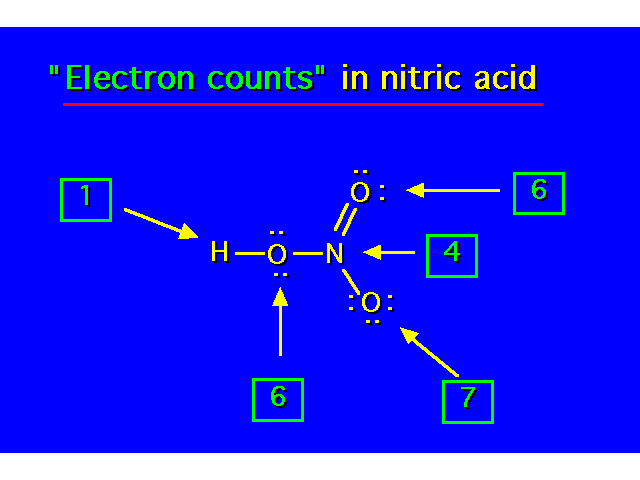
Covalence Numbers: The number of covalent bonds in which an atom participates when it has no formal charge.
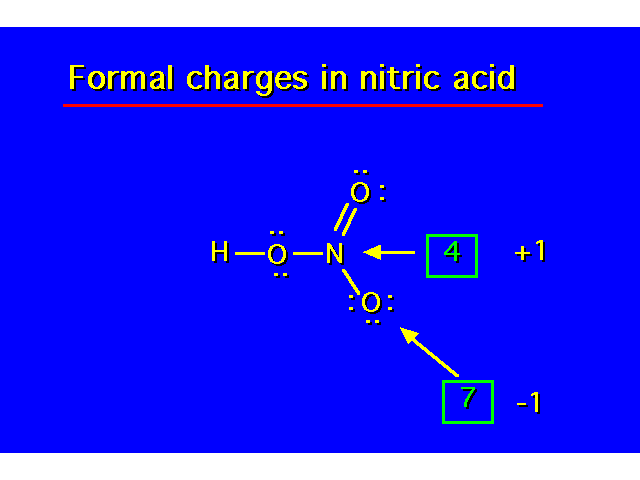
Formal charges will result when an atom in a molecule does not have its optimum covalence number. *Boron does not obey the octet rule. In neutral compounds, it possesses only 6 electrons. |  | ||||||||||||||||||||||||||||
Nitrogen needs to own 5 valence electrons for neutrality (each lone pair electron belongs to N and one in each covalent bond will belong to N. In this example, N owns only 4 valence electrons so F.C.= +1 Boron needs to own 3 valence electrons for neutrality. In this example, B owns 4 valence electrons so F.C.= -1. | 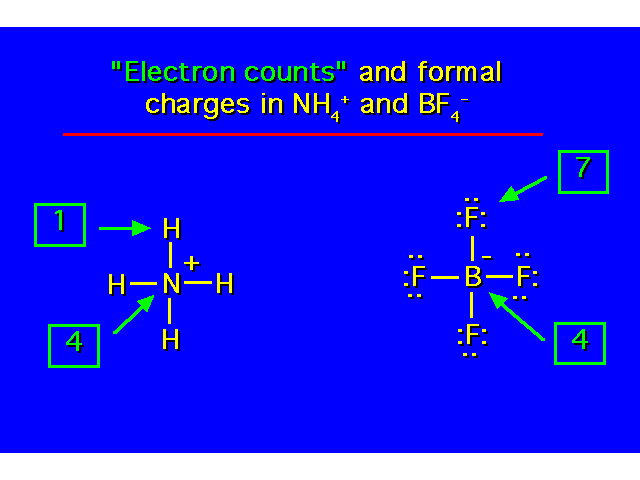 | ||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
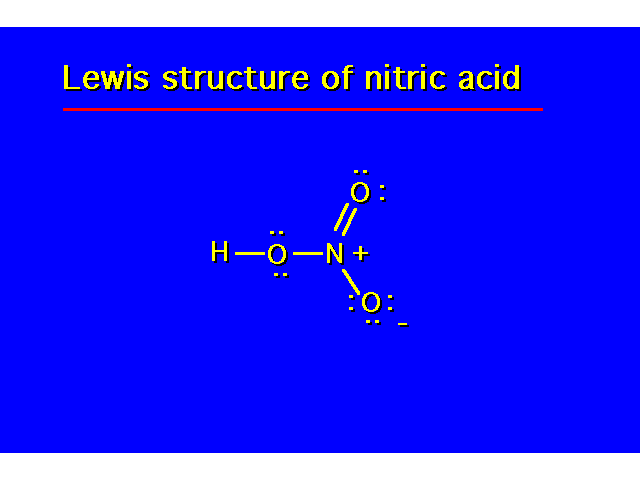 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
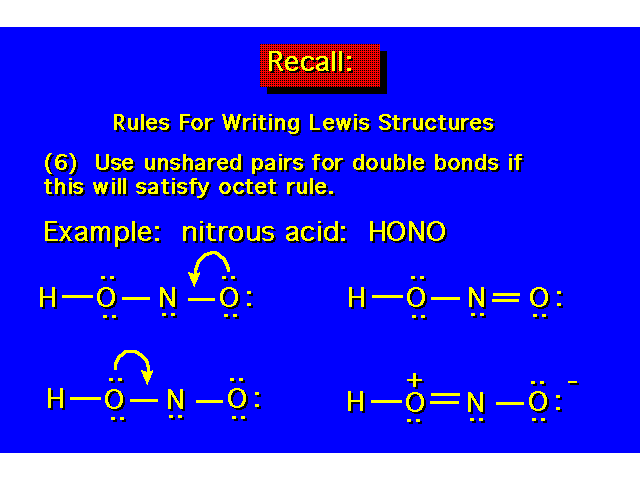 | |||||||||||||||||||||||||||||
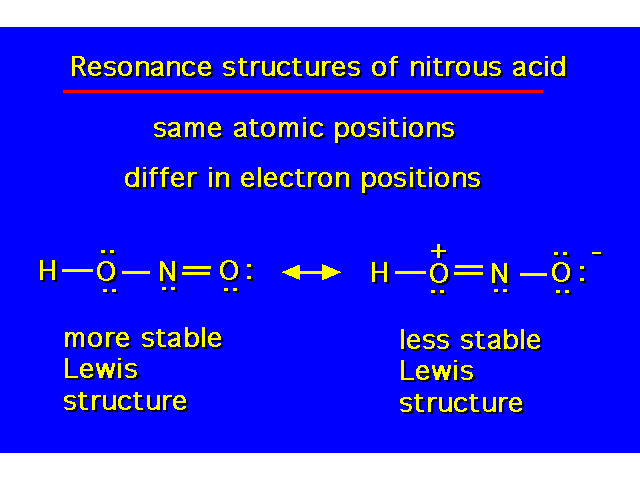 | |||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||
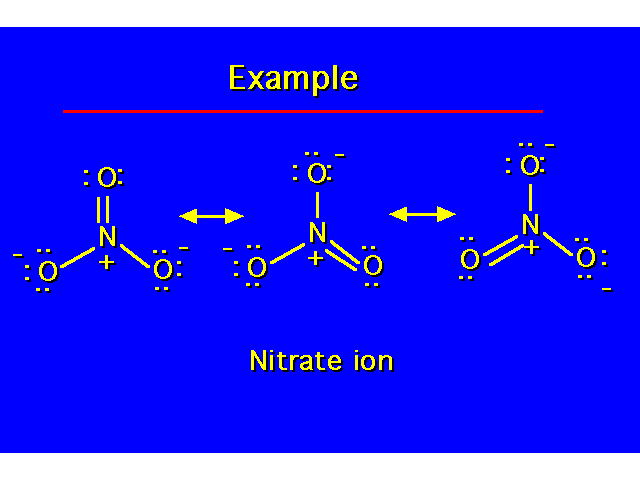 | |||||||||||||||||||||||||||||
 Return to the Syllabus
Return to the Syllabus