The Oxidation States of Vanadium
Purpose
The goal of this experiment is to prepare the four common oxidation states of vanadium, separate them using ion-exchange chromatography and characterize them using chemical and spectroscopic techniques.
Background Information
 Vanadium
is a d-transition metal found in Group VA of the Periodic Table. Vanadium
was first discovered by the Spanish Mineralogist Andres Manuel del Rio
in 1801 while experimenting with a mineral obtained from a mine near
Hidalgo in Northern Mexico. He prepared a number of colored salts from
this "brown lead" which were similar to salts of chromium.
He named his new element erythronium which means red after observing
that most of his salts turned red upon heating. A French chemist named
Collett-Desotils disputed his claim of discovering a new element insisting
that his new element was nothing more than impure chromium.
Vanadium
is a d-transition metal found in Group VA of the Periodic Table. Vanadium
was first discovered by the Spanish Mineralogist Andres Manuel del Rio
in 1801 while experimenting with a mineral obtained from a mine near
Hidalgo in Northern Mexico. He prepared a number of colored salts from
this "brown lead" which were similar to salts of chromium.
He named his new element erythronium which means red after observing
that most of his salts turned red upon heating. A French chemist named
Collett-Desotils disputed his claim of discovering a new element insisting
that his new element was nothing more than impure chromium. Del Rio, believing he was incorrect, withdrew his claim of discovery.
Thirty years later, the Swedish chemist Nils Sefström isolated
a new oxide while experimenting with some iron ores. He named this new
element Vanadium in honor of the Scandanavian goddess of beauty, Vanadis,
because of its varied beautiful, multicolored compounds.
Del Rio, believing he was incorrect, withdrew his claim of discovery.
Thirty years later, the Swedish chemist Nils Sefström isolated
a new oxide while experimenting with some iron ores. He named this new
element Vanadium in honor of the Scandanavian goddess of beauty, Vanadis,
because of its varied beautiful, multicolored compounds.
Vanadium
naturally occurs in about 65 different minerals, including vanadinite,
the ore of lead and vanadium, from which Del Rio first isolated it.
In the U.S. it is most commonly found in sandstones which are rich in
uranium ores. Vanadium is also  found
carbon-rich deposits such as coal, oil shale, crude oil, and tar sands.
While minor amounts of vanadium are found in the human body, its biological
purpose is unknowns. Vanadium is an important element in sea squirts
of the ascidian family. These marine filter feeders concentrate vanadium
in their bodies to a level one million times higher than the concentration
of vanadium in seawater.
found
carbon-rich deposits such as coal, oil shale, crude oil, and tar sands.
While minor amounts of vanadium are found in the human body, its biological
purpose is unknowns. Vanadium is an important element in sea squirts
of the ascidian family. These marine filter feeders concentrate vanadium
in their bodies to a level one million times higher than the concentration
of vanadium in seawater.
Vanadium is used in making specialty steels, rust resistant and high speed tools.
Vanadium's ground state electron configuration is [Ar] 3d34s2. When transition elements ionize, they lose their valence s electrons before losing their d electrons. Vanadium has 5 valence electrons that can be lost. One of the characteristics of transition metal is their ability to adopt multiple oxidation states. Vanadium exhibits four common oxidation states +5, +4, +3, and +2 each of which can be distinguished by its color.
|
Oxidation State
|
Ion
|
Color
|
|
+5
|
VO3- or VO2+
|
Yellow
|
|
+4
|
VO2+
|
Blue
|
|
+3
|
V3+
|
Green
|
|
+2
|
V2+
|
Violet
|
The ease with which the different
oxidation states of vanadium can be interconverted has led to its usage
in a vanadium flow
battery. In the vanadium redox battery (VRB), each half-cell is
composed of a vanadium redox couple. At the anode VO2+ ions are converted
to VO2+ ions and when electrons are removed from the positive terminal
of the battery. At the cathode, electrons convert the V3+ ions into
V2+. 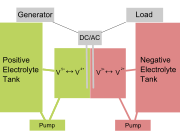 The
electrolytes of the two half-cells are separated by an ion exchange
membrane. In this battery, half-cells are connected to storage tanks
and pumps allowing very large volumes of the electrolytes can be circulated
which allows them to be very high capacity batteries for large scale
power storage. These batteries are being used to store electricity from
solar energy collectors and wind mills. The battery has also been used
to power vehicles. One interesting feature of the VRB is its ability
to be recharged by either conventional means or by exchanging spent
solutions for fresh ones at suitable refueling stations. A golf cart
that runs on VRB power has been developed for test purposes. Learn more
about the the Vanadium Redox Battery here.
The
electrolytes of the two half-cells are separated by an ion exchange
membrane. In this battery, half-cells are connected to storage tanks
and pumps allowing very large volumes of the electrolytes can be circulated
which allows them to be very high capacity batteries for large scale
power storage. These batteries are being used to store electricity from
solar energy collectors and wind mills. The battery has also been used
to power vehicles. One interesting feature of the VRB is its ability
to be recharged by either conventional means or by exchanging spent
solutions for fresh ones at suitable refueling stations. A golf cart
that runs on VRB power has been developed for test purposes. Learn more
about the the Vanadium Redox Battery here.
In this experiment, you will start with the +5 oxidation state of vanadium, generate the other oxidation states by redox reactions, and separate them using ion exchange chromatography. Ammonium metavanadate, NH4VO3 will be the source of the +5 oxidation state. Treatment of the ammonium metavanadate with hot hydrochloric acid partly reduces the vanadium to the +4 oxidation state in the form of the VO2+ ion. The two species are separated using an ion exchange resin. The VO2+ ion is then treated with mossy zinc generating the V3+ and V2+ which also are separated by ion exchange chromatography.
Ion Exchange Chromatography (IEC)
Ion exchange chromatography
is a form of liquid chromatography in which retention of the materials
in a mixture is predominately controlled by electrostatic interactions
between the charges present in the molecules or ions of the solute and
opposite charges present in the stationary phase. In order to separate
a mixture of cations, the stationary phase must contain immobilized
anions with which the cations can interact. The stationary phase is
usually an organic polymer fabricated into small 1-2 mm beads onto which
sites where ions are easily trapped and released. The polymer used in
our separation is a divinylbenzene cross-linked polystyrene that has
been modified by adding sulfonic acid groups to the benzene rings by
sulfonation of the polymer and is classified as a strongly acidic resin.
The properties of the resin can be changed markedly by altering the
amount of cross-linking in the polymer. The amount of cross-linking
in these polymers is dependent on the proportions of the monomers used
in the polymerization step and typically ranges from 2-16%. The resin
used in our separation is AG50W-2X where the 2X refers to 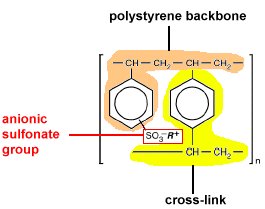 the
amount of cross-linking. In generating this resin, 2% divinyl benzene
is added to the styrene monomer used in the polymerization step. Sulfonation
introduces sulfonic acid groups (-SO3H) inside the polymer
particles as well as onto the surface. The more highly cross-linked
the polymer, the harder it is to add sulfonic acid groups into the particles.
The more highly cross-linked, the lower the capacity of the resin. In
it's native form the R+ is the hydrogen and the group is a sulfonic
acid group. During the chromatographic separation, the hydrogen is replaced
by other cations. The stronger the electrostatic interaction between
a cation and the SO3
the
amount of cross-linking. In generating this resin, 2% divinyl benzene
is added to the styrene monomer used in the polymerization step. Sulfonation
introduces sulfonic acid groups (-SO3H) inside the polymer
particles as well as onto the surface. The more highly cross-linked
the polymer, the harder it is to add sulfonic acid groups into the particles.
The more highly cross-linked, the lower the capacity of the resin. In
it's native form the R+ is the hydrogen and the group is a sulfonic
acid group. During the chromatographic separation, the hydrogen is replaced
by other cations. The stronger the electrostatic interaction between
a cation and the SO3
Many natural water sources in the Willamette Valley are hard water sources meaning that they have significant amounts of magnesium and calcium ions along with dissolved iron. These ions react are responsible for deposits that build-up in pipes and that stain sinks, showers and tubs. If this hard water is passed through a column containing and ion exchange resin having sodium ions in its active sites, the undesired ions replace the sodium ions and are immobilized on the resin lowering the concentration of these ions in the water. Learn more about ion exchange and water systems here.
Lab Procedure
Part I. Preparation of the Chromatography Column
- Push a small plug of glass wool down to the stopcock of a 25-mL buret.
- Close the stopcock and fill the buret with an aqueous slurry of AF50W-X2 cation exchange resin (100-200 mesh, H+ form). Allow the resin to settle. Open the stopcock and continue adding slurry to the column until the column of settled resin is 5-6 inches high. Be sure to keep the level of liquid above the top of the resin during the column packing process. Make sure the column is tightly packed. Wash the column of resin with distilled water until the eluate is clear.
Part II. Separation #1
- In the fume hood, add 2.0 mLconcentrated hydrochloric acid to 0.2 g of NH4VO3 in a large test tube. Heat the mixture in a boiling water bath for 5 minutes. Add 10 mL distilled water and mix well. The solution should change from its original yellow color to a bright green.
- Carefully pour the green solution onto the top of the cation exchange column. Drain the column via the stopcock until the level of the liquid falls just to the top of the resin.
- Fill the buret with 0.4 M HCl. Open the stopcock to begin chromatography. Add 0.4 M HCl as needed to keep the buret full. Two bands will appear on the column. The upper band, although blue, will appear to be green as seen through the orange color of the resin. The lower band is yellow which is hard to see on the column because of the resin color. When the eluate from the column becomes yellow, begin collecing fractions of 5-6 mL in test tubes. When all of the yellow species has been collected from the column (the eluate becomes green or blue), change the eluting solvent to 1.0 M HCl. Continue collecting fractions until all of the second band has been eluted. Combine all the blue fractions together (should be approximately 25-30 mL). Combine all the darkest yellow fractions together in another container. Dispose of any fractions that are yellow-green or blue-green as they are mixtures of the two ions. Be sure to leave a solvent head on the column.
Part III. Reduction and Separation #2
- Transfer 15-20 mL of the blue solution to a 25-mL Erlenmeyer flask containing about 3 g of mossy zinc. Put a cork into the flask and swirl for 5-10 minutes until the color becomes lavender in color and no longer changes. The color should become green before turning lavender.
- Decant the solution and pour it onto the chromatography column. Drain the column via the stopcock until the level of the liquid falls just to the top of the resin.
- Elute with 1.0 M HCl. Distinct green and lavender bands should appear. Collect in ~5 mL fractions in testtubes. After all of the lavender species has been collected, change the eluting solvent to 3.0 M HCl and elute the green species. Combine solutions of the same species.
Part IV. Characterization of Products.
- Place a 1 mL sample of each solution into a different test tube and add 0.001 M KMnO4 dropwise. Note which solutions decolorize the permanganate and offer an explanation for your results.
- Place a 1 mL sample of each solution into a different test tube and add iodine water dropwise. Note which solutions decolorize the iodine and offer an explanation for your results.
- Acquire a visible spectrum of each of the four ions, noting the absorbance maxima for each oxidation state. You need to find the literature values for the absorbance maxima of the ions and compare your results to the literature.
- Reduce a portion of the green solution from Separation #1 directly with mossy zinc to V(II). Exposure of the resulting solution to air will oxidize V(II) to V(III).
- Wash the resin by passing 20-30 mL of water through the column. Remove the resin from the buret and collect the resin by gravity filtration. Return the resin to the original container.
Report
You will write a formal, typed laboratory report for this experiment. Guidelines for this report can be found here.
Additional References
General informationon the chemistry of vanadium can be found in the following sources:
- Comprehensive Inorganic Chemistry, Vol. 3, Chapter 34. (in WOU library)
- Cotton & Wilkinson, Advanced Inorganic Chemistry, Chapter 25.
Electrochemistry:
- Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions (in WOU library)
- Any general chemistry text will have a section on balancing redox equations.
- Pourbaix diagrams are useful in predicting the stabilities of various species. A discussion of these diagrams can be found in J.Chem. Educ. 1969, 46, 90.
- An explanation of how to get information from a Pourbaix diagram can be accessed here.