The Synthesis
of "Bouncing Putty"
A Cross-Linked
Silicone Polymer
![]()
Purpose
The goal of this experiment is to synthesize bouncing putty, a cross-linked silicone polymer, and study its unique properties.
Background Information
Silicon is the second most abundant element
in the earth's 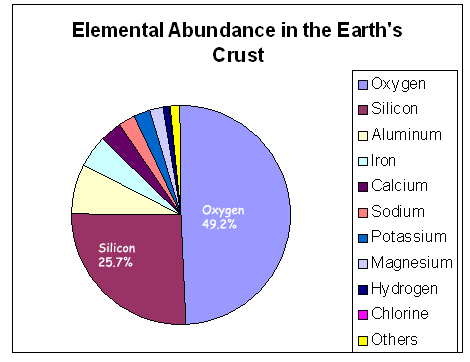 crust
and is a major component of many minerals (silicates).
It is a member of the carbon family and is the family member directly
below carbon on the periodic table.
crust
and is a major component of many minerals (silicates).
It is a member of the carbon family and is the family member directly
below carbon on the periodic table.
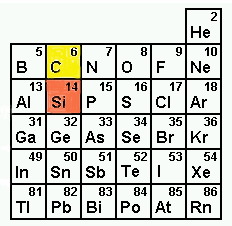
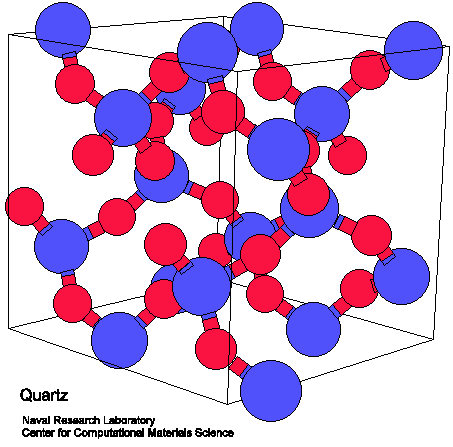
Despite their proximity on the periodic table, the chemistry of silicon and carbon are quite different. For example, silica SiO2 (the primary structure of quartz) has a diamond crystalline structure in which each silicon bonds to four neighboring oxygen atoms to form an extended lattice. In contrast, carbon dioxide CO2 exists as discrete molecules. Although carbon has the ability to form strong pi bonds with other carbons or oxygen, pi bonds between silicon and other atoms are unstable. Silicon forms polymers with very strong single bonds to multiple oxygen atoms rather than discrete molecules possessing multiple bonds to single oxygens.
![]()

![]()
Polyorganosiloxanes have empirical formulas of R2SiO and might be expected to be similar in structure and chemistry to organic ketones, R2CO. These molecules were originally named silicones on the belief that they were silicon analogs of ketones.

In reality, silicones have little in common with ketones. Ketones exist as discrete molecules possessing the carbon-oxygen double bond, while silicones consist of polymeric chains with an alternating silicon-oxygen backbone having two alkyl groups bonded to each silicon atom.
Although there is no similarity between the structure of organic ketones and silicones,the name stuck so these polyorganosiloxane polymers are commonly called silicones.
Silicones are viscoelastic materials. They behave like viscous liquids
at high temperature or when allowed to flow over a long period of time.
They behave like elastic solids or elastomers
at low temperature.The elastic behavior stems from the very 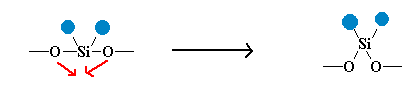 flexible
silicon-oxygen bonds whose angle can easily open and close in a scissor-like
fashion. When the molecular weight is high, these flexible chains become
loosely entangled which imparts a high level of viscoelasticity.
flexible
silicon-oxygen bonds whose angle can easily open and close in a scissor-like
fashion. When the molecular weight is high, these flexible chains become
loosely entangled which imparts a high level of viscoelasticity.
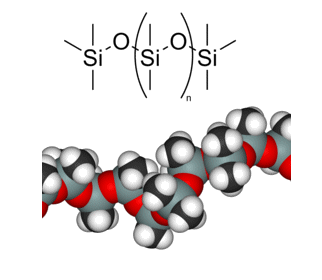 Polydimethylsiloxane
(PDMS), which has the repeating
Polydimethylsiloxane
(PDMS), which has the repeating
(CH3)2SiO unit,
is the most common of the organosilicones. Altering the number of repeat
units (value of n) in the chain and the degree of cross-linking which
ties multiple polymer chains together generates polymers possessing different
physical properties. Fluids, emulsions, lubricants, resins, elastomers
or rubbers are different classes of commercially important PDMS products.
Since silicon-chlorine bonds are very susceptible to cleavage by water,
polydimethylsiloxane can be synthesized by hydrolyzing dichlorodimethylsilane.
n [Si(CH3)2Cl2] + n H2O ->[Si(CH3)2O]n + 2n HCl
The initial hydrolysis reaction exothermically generates a silanol Si(CH3)2(OH)2 which readily condenses through loss of water to form the siloxane polymer. Since dichlorodimethylsilane is bifunctional (has two chlorines), the chain is able to propagate in two directions generating high molecular weight polymers which retain some residual hydroxyl groups. These residual hydroxyl groups react with boric acid B(OH)3 to form Si-O-B linkages between polysiloxane chains. Since boric acid is trifunctional, a single boron has the ability to join three polysiloxane chains together. This joining of chains is called cross-linking. Cross-linking produces a high molecular weight polymer that is a soft, pliable gum with very interesting chemical properties.
A Little History
During World War II, there was a lot of interest in developing an inexpensive substitute for synthetic rubber. James Wright, an engineer at General Electric who was working on these substitutes, added boric acid to silicone oil (PDMS) to form a product (view Wright's patent) that could be stretched farther than rubber and bounced 25% higher than rubber. This new substance also transferred ink from newsprint. Although Dr. Earl Warrick of Dow Corning synthesized the same polymer (view Warrick's patent) at about the same time, Wright gets most of the credit for inventing the polymer because of the events which followed his discovery.
For a number of years, the polymer termed "nutty putty"
at General Electric remained a curiosity. It was played with by the company's
chemists but seemed to have no commercial use (read the January
1945 Popular Science article). In 1949, a marketing expert named Peter Hodgson was introduced to nutty
putty at a party and saw the marketing potential it had as a toy. As the
story goes, Hodgson, who was unemployed
In 1949, a marketing expert named Peter Hodgson was introduced to nutty
putty at a party and saw the marketing potential it had as a toy. As the
story goes, Hodgson, who was unemployed at the time borrowed $147 to buy a supply and the marketing rights of
the putty. Since it was the Easter season, he packaged it in one ounce
lumps in plastic eggs which he sold for $1. He needed a marketing name
and called it "Silly Putty" for it's principal silicone ingredient.
The road to Silly Putty fame was a bumpy one.....Hodgson was told by the
experts that his toy would never sell, and just as he was beginning to
find a market, the Korean War spurred the government to place restrictions
on raw materials including the silicone needed to make the putty. When
the restrictions were lifted, Hodgson, still believing in his putty, made
another attempt at marketing it. Although Silly Putty was intended originally
to amuse adults, by 1955, it had became popular as a children's toy (view
the first
Silly Putty commercial). By the late fifties and early sixties, it had become a multi-million
dollar seller (view a an early
television commercial , a
comic book ad, a timeline of Silly Putty history
, university student project, short video "Silly Putty Invention of War"). At the time of his death in 1976, Hodgson's estate
was worth over $140 million - all due to the silicone putty that had no
practical use!
at the time borrowed $147 to buy a supply and the marketing rights of
the putty. Since it was the Easter season, he packaged it in one ounce
lumps in plastic eggs which he sold for $1. He needed a marketing name
and called it "Silly Putty" for it's principal silicone ingredient.
The road to Silly Putty fame was a bumpy one.....Hodgson was told by the
experts that his toy would never sell, and just as he was beginning to
find a market, the Korean War spurred the government to place restrictions
on raw materials including the silicone needed to make the putty. When
the restrictions were lifted, Hodgson, still believing in his putty, made
another attempt at marketing it. Although Silly Putty was intended originally
to amuse adults, by 1955, it had became popular as a children's toy (view
the first
Silly Putty commercial). By the late fifties and early sixties, it had become a multi-million
dollar seller (view a an early
television commercial , a
comic book ad, a timeline of Silly Putty history
, university student project, short video "Silly Putty Invention of War"). At the time of his death in 1976, Hodgson's estate
was worth over $140 million - all due to the silicone putty that had no
practical use!
In 1977, Binney & Smith Inc., the Crayola Crayon company, acquired the rights to Silly Putty and continues to sell it today (see how it is processed). In March 2001, Silly Putty was inducted into the Toy Hall of Fame. Even Chemical and Engineering News, the weekly news magazine of the American Chemical Society, wrote a story for the 50th Anniversary of Silly Putty.
Generic "Silly Putty" (can't technically be called Silly Putty as that is a trademark owned by Binny & Smith) is still produced by Dow Corning as Dow Corning 3179 Dilatant Compound (MSDS). There is more in that little plastic egg than the cross-linked polymethylsiloxane polymer. Additives are used to make the gooey polymer into the toy with which we are all familiar (fact sheet, ingredients list). You can buy this generic material in bulk for $400/50 pounds from Dow Corning or 5 pound bulk. It costs about $67.75/pound out of the egg. So what do you do with 50 pounds of Silly Putty? Watch this video to find out.
Properties
Bouncing putty has interesting properties. If you pull it slowly, it
stretches. It can be molded into all sorts of shapes like modelling clay
but over time will flow  (a video example).
If you pull it quickly, it breaks. It bounces like a rubber ball, but,
under very high impact conditions, it shatters. The secret to the behavior
of bouncing putty lies in the cross-linking.
(a video example).
If you pull it quickly, it breaks. It bounces like a rubber ball, but,
under very high impact conditions, it shatters. The secret to the behavior
of bouncing putty lies in the cross-linking.
In the polymer, the boron links polysilicone chains together. However, this is a dynamic linkage. Thermal motion in the molecule can break the linkage allowing it to slide to a new position along the chain where it re-attaches. A pair of chains can move past each other when the cross-linking bonds are temporarily broken. The putty is able to be stretched when deformation occurs on a time scale that is slower than the cross-link bond break-and-reconnect rate. If the material is deformed too rapidly, it acts as a rigid stubstance because there is not time for the cross-links to be reconnected after they break. If the deformation is only somewhat faster than the breaking-reconnecting rate, the chains will move some then snap back elastically giving the bouncing behavior. If the deformation is very rapid, the cross-links break but do not reconnect separating the chains, snapping the sample into pieces. Hitting a sample with a hammer causes it to shatter. Warming a sample of putty causes the disconnection-reconnection rate to increase; freezing the sample, decreases the rate.
Experimental Procedure
Part I. Preparation of Polydimethylsiloxane
This part of the procedure must be carried out in the fume hood because the hydrolysis reaction releases toxic hydrogen chloride gas.
 Place
20 mL of dichlorodimethylsilane (MSDS)
into a dry 250 mL Erlenmeyer flask fitted with a rubber stopper and a
stirring bar. The dichlorodimethysilane is very sensitive to moisture
in the atmosphere so make your transfer quickly. Add 40 mL of anhydrous
diethyl ether and hydrolyze the silane by dropwise addition of 40 mL of
water with constant stirring. The water must be added slowly at first,
or the exothermic evolution of the HCl gas will be extremely vigorous
boiling your ether away. Have an ice water bath available to slow your
reaction if the hydrolysis becomes too vigorous. After adding about 10
mL of water, you should be able to increase the rate of addition. When
all the water has been added, let the mixture stir for a few minutes and
then transfer it to a 250 mL separatory funnel. Separate the layers discarding
the aqueous layer. Wash the ether layer with three 100 mL portions of
1 M sodium carbonate solution to neutralize residual acid (remember that
neutralization of an acid by carbonate solution evolves carbon dioxide
gas!) After washing the ether layer with the first 100 mL portion of carbonate
solution, add 10 mL of diethyl ether to replace any solvent that was evaporated
during the exothermic neutralization reaction. Wash the ether layer with
100 mL of water and dry over anhydrous magnesium sulfate in a stoppered
Erlenmeyer flask. When the solution is dry, decant it into a tared flask
and evaporate the ether using either a warm water bath or the rotary
evaporator. Record the yield of your dimethylsilicone oil.
Place
20 mL of dichlorodimethylsilane (MSDS)
into a dry 250 mL Erlenmeyer flask fitted with a rubber stopper and a
stirring bar. The dichlorodimethysilane is very sensitive to moisture
in the atmosphere so make your transfer quickly. Add 40 mL of anhydrous
diethyl ether and hydrolyze the silane by dropwise addition of 40 mL of
water with constant stirring. The water must be added slowly at first,
or the exothermic evolution of the HCl gas will be extremely vigorous
boiling your ether away. Have an ice water bath available to slow your
reaction if the hydrolysis becomes too vigorous. After adding about 10
mL of water, you should be able to increase the rate of addition. When
all the water has been added, let the mixture stir for a few minutes and
then transfer it to a 250 mL separatory funnel. Separate the layers discarding
the aqueous layer. Wash the ether layer with three 100 mL portions of
1 M sodium carbonate solution to neutralize residual acid (remember that
neutralization of an acid by carbonate solution evolves carbon dioxide
gas!) After washing the ether layer with the first 100 mL portion of carbonate
solution, add 10 mL of diethyl ether to replace any solvent that was evaporated
during the exothermic neutralization reaction. Wash the ether layer with
100 mL of water and dry over anhydrous magnesium sulfate in a stoppered
Erlenmeyer flask. When the solution is dry, decant it into a tared flask
and evaporate the ether using either a warm water bath or the rotary
evaporator. Record the yield of your dimethylsilicone oil.
Part II. Cross-linking the Polymer
With stirring using a stirring rod, add 5% by weight of boric acid to the flask. Your oil should become very viscous so mix it well. Heat the mixture using a 180 oC oil bath for 1.5-2.5 hours. The longer the reaction time, the better your product should be. At the end of the cross-linking reaction, allow the flask to cool and collect the product by scrapping it from the flask with a spatula. Kneed the gummy substance to get a putty like consistency.
Part III. Testing the Product
Perform the following tests on samples of your putty and on a sample of commercial Silly Putty:
- Roll it into a ball and test its bouncing properties
- Test its stretching properties by first pulling it slowly and then by pulling it sharply
- Place a sample on a hard surface and apply a constant pressure with
your thumb

- Place a small sample on a hard, non-breakable surface and hit it with a hammer
- Repeat the pulling and bouncing tests on putty that has been chilled and warmed
- If you press a sample on a newspaper, does ink transfer to the sample?
- Place a small sample on the whiteboard and mark its location. Leave it on the whiteboard for a number of days and then compare the before and after locations.

In your notebook, compare the physical qualities of your putty to the commercial putty as well the results of the tests on each. Provide an explanation for the behaviors you observe in each test for the putty samples.
