The Cascades are a 30-70 mile wide mountain range that extends about 700 miles from British Columbia to California. The crest of the range lies from 100 to 150 miles from the Pacific Ocean. The Cascade Range is actually composed of two parallel chains -- an older, deeply eroded region called the Western Cascades and a younger region further to the east called the High Cascades. The Western Cascades increase in altitude from about 1700 feet on the western margin to about 5,800 feet on their eastern edge. The High Cascades average 5,000-6,000 feet in altitude with snow covered peaks in excess of 11,000 feet. The Western Cascades receive 60 to 100 inches of precipitation annually which causes deep weathering of geologic features and supplies the headwaters of several major west-flowing rivers that drain the range such as the Clackamas, Santiam, and McKenzie which eventually empty into the Willamette River and the Umpqua and Rogue which flow directly into the ocean.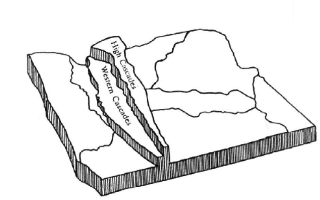
The Cascade Range was formed by a series of volcanic episodes beginning about 40 million years ago. These volcanic processes shaped the Western Cascades until about 5 million years ago when two major volcanic events formed the High Cascades. Thus, the range is composed almost entirely of volcanic rocks. These rocks are formed by the melting and recrystallization of magma during tectonic cycling (the theory of plate techtonics 1,2). Heat flow from the Earth's interior induces convection currents that rise toward the surface and causes mantle material to rise. As this material rises, the pressure on it decreases causing the minerals to melt forming magma. The magma cools as it nears the surface forming the igneous rocks of the earth's crust. New crust is continuously formed in this process. Simultaneously, segments of the crust are drawn back into the mantle (subduction -- a process happening off the Oregon coast).
By far, the two most abundant chemical elements in the earth's crust are oxygen (46.6%) and silicon (27.7%; next is aluminum at 8.1%). Silicates are the most common constituents as much as 40-80 percent by weight, in igneous rocks. These silicates often contain one or more of the elements Al, Fe, Ca, Na, K, or Mg. Although iron and aluminum are relatively abundant in the earth's crust, most of the metals deemed essential to our industrialized civilization are highly dispersed throughout the crust, i.e., Cu (0.0055%); Pb (0.0013%); Ag (0.000007%) and Au (0000004%). During the geologic processes that form mineral deposits, many chemical changes occur. Through some of these changes, elements that were dispersed through large volumes of rock can be collected and concentrated in a particular location to form ores. By definition, an ore is a mineral accumulation that can be extracted at a profit for refinement and industrial use. For example, galena (PbS), the most common ore of lead, contains about 86% lead. Unfortunately, ores like galena are rarely found in massive clumps that can be extracted directly. Rather, these ores often are found in mixtures with other minerals that have no commercial value (gangue minerals). Thus, mineral deposits are classified according to grade, a measure of the concentration of the element in the ore. The cut-off grade is the minimum concentration that will allow the element to be extracted at a profit. In addition to the grade, a mineral deposit must be of a minimum size (given in weight of ore usually in tonnes). In other words, the deposit must contain enough ore to be profitable. The mining operation of a deposit will continue as long as the ore can be extracted at a profit. When the operation is no longer profitable, the mine is usually abandoned.
Placer gold was first discovered in the Cascades at Sharps Creek in 1858 (Bohemia district) and lode gold in 1863. Despite the wild nature of the locality, the Bohemia Gold and Silver Mining District was organized in 1867 and has produced in excess of one million dollars worth of gold. Gold and silver deposits have been found to exist in a 25-30 mile wide, north-south belt in the Western Cascades of Oregon. Five major mining areas are located in this belt -- the North Santiam district in Clackamas and Marion counties, the Quartzville district in Linn County, the Blue River district of Linn and Lane counties, and the Fall Creek and Bohemia districts in Lane County.
![]() Click here to view the labelled area
Click here to view the labelled area
Deposits of lead, zinc and copper in the forms of pyrite, galena, sphalerite and chalcopyrite in addition to gold and silver are found in this region.
These ores are found in either veins or porphyry deposits formed when fractured areas of volcanic rock were invaded by mineral rich hydrothermal solutions at temperatures of 250 to 350 degrees F. Hydrothermal solutions are sodium-calcium chloride brines containing magnesium and potassium salts along with small amounts of other elements. These brines are effective solvents for many sulfide and oxide minerals and can dissolve and transport native metals such as gold and silver. During vein formation, the hydrothermal solution flows through an open fissure depositing its dissolved minerals.
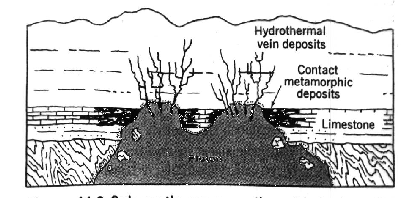 Vein Formation
Vein Formation
When a hydrothermal solution flows into a region of shattered rock, the shattered rock becomes a permeable medium for the solution producing in porphyry deposits. Porphyry copper deposits contain what is known as disseminated mineralization -- a large volume of shattered rock containing a network of tiny quartz veins of chalcopyrite spaced only a few centimeters apart. Pyrite often accompanies these deposits. Although the concentration of ore per unit of volume is less in porphyry deposits than in vein deposits (typically only 0.5 to 1.5% copper by weight), these deposits tend to be extensive. In fact, more than 50 percent of all mined copper comes from porphyry deposits. Porphyry copper deposits are often associated with stratovolcanoes and are a common mineralization found in the volcanic region that rings the Pacific Ocean basin.
Weathering processes have deeply eroded the Western Cascades exposing the ore-bearing rocks near the valley floors. The North Santiam mining district contains the most rugged terrain of all the five mining districts. Most of the mines are located less than 500 feet above the Little North Fork of the Santiam or one of its tributaries. Quite a bit of mining activity occurred in this North Santiam district from about 1903 into the 1930s with copper, zinc and lead being the primary metals extracted. The Amalgamated Mine, located about 26 miles by road from the town of Lyons, shipped about 43 tons of crude ore to the smelter. The vein matter was composed of sphalerite, galena and chalcopyrite with very little associated pyrite. Sulfides were also disseminated irregularly through the vein matter with occurences of large lumps of almost solid sulfur. The gangue material was a soft greenish mass of chlorite (iron, magnesium,or aluminum silicates). The table below shows a typical analysis of ore obtained from this site.
| Element | Unit of Measure | Amount |
|---|---|---|
| Gold | Trace | |
| Silver | oz. to ton | 4.1 |
| Lead | percent | 19.4 |
| Copper (wet) | do | 1.8 |
| Copper (insoluble) | do | 3.8 |
| Iron | percent | 4.3 |
| Zinc | do | 35.5 |
| Sulfur | do | 23.0 |
| Arsenic | do | 0.2 |
| Antimony | do | 0.1 |
According to a story which appeared in the April 30, 1996 issue of The Oregonian (p. B02), President Clinton approved funding of $300,000 to clean up this site. Senator Mark Hatfield, R-OR, was a major proponent of the initiative. According to the iniative, mine tailings stored under a tarp near Battle Ax Creek would be cleaned up by the U.S. Forest Service. These residues, about 5,500 cubic yards of material, were moved to this site for temporary storage in 1991 when it was feared that the rotten retaining wall behind which this waste was stored would fail releasing the tailings into Battle Ax Creek. In 1992, the EPA determined that the site did not pose a significant enough health hazard to warrant further cleanup efforts so this temporary pile became permanent, forgotten until flooding during February 1996 brought the water of Battle Ax Creek to the edge of the toxic pile. Any potential contamination of Battle Ax Creek is a concern. Battle Ax Creek flows into Opal Creek, which in turn empties into the North Santiam River, a source of drinking water for about 150,000 Salem-area residents.
The U.S. Forest Service devised a plan to move the toxic waste to the original mine site about a half-mile away from its present location, creating a landfill in which the tailings would be mixed in layers with dry cement and buried in a rubber-lined hole at a depth of 15 to 20 feet. The hole would be overlayed with 2 feet of gravel and the surface planted with grass. This proposed landfill site lies about 300 feet from Opal Creek. The landfill plan has a price tag of $663,000 while transporting all of the material out of the forest to another area would cost at least $1.7 million excluding the cost of road improvements. This plan is supported by the U.S. Forest Service, the Oregon Department of Environmental Quality and the environmental group Friends of Opal Creek owners the proposed burial site. According to the plan, the less than one acre burial site would be deeded to the U.S. Forest Service. Officials of the City of Salem along with other environmental groups like the Eugene-based Native Forest Council oppose this plan. Friends of Opal Creek are in favor of the plan because the proposed landfill site is an area of the forest already scarred by the former mining operation. Choice of any other site would require the clearing away of trees. This group contends that trucking the waste out of the forest to another site would compromise the historic integrity of the area which is explored annually by thousands of hikers who come to experience an old growth forest. Engineers from the Department of Environmental Quality insist that no health or environmental problems would result from flooding or failure of the burial sites rubber lining. According to Michael McCann, a DEQ engineer, "The stuff just doesn't leach very much, and the amount that leaches is so small compared to the flow in Opal Creek. We wouldn't be able to detect a difference"(quoted from the July 21, 1996 issue of The Oregonian, p.B03). One issue concerning Salem officials is the fact that the plan does not include any provisions for monitoring the leachate or the water quality of Opal Creek. As of this writing, October 1996, the tailings pile remains undisturbed under its tarp along Battle Ax Creek.
![]()