Polysilicates
The Orthosilicate Ion, SiO44-
The orthosilicate ion is not present in a wide variety of minerals. It is a very strong base which will not persist in aqueous solution. In nature it is found in combination with acidic cations in insoluble salts.| phenacite | Be2SiO4 | |
| willemite | Zn2SiO4 | |
| zircon | ZrSiO4 | |
| garnet | (M2+)3(M3+)2(SiO4)3 | M2+ = Ca, Mg, Fe M3+ = Al, Cr, Fe |
Oligomeric Polysilicates
Polymeric silicate structures require bridging (2-coordinate) oxygens. In order to make room for a bridging oxygen, an oxide must be removed from the "receiving" silicon.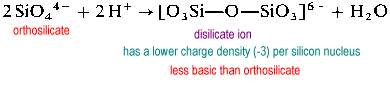
The disilicate ion is uncommon in nature. It is found only in the rare mineral thortveitite, Sc2Si2O7. Larger structures such as trisilicate and tetrasilicate are extremely rate.
Cyclic Oligomeric Polysilicates
Instead of forming long open chain structures, the ends of the chains will link eliminating oxide ions.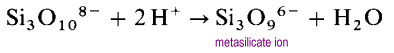
The metasilicate ion is an oligomer of the unknown SiO32- ion. In these structures each silicon possesses two bridging and two terminal oxygen atoms. There is a -2 charge density per silicon atom. The most common cyclic polysilicates are the cyclic trimers, (SiO3)36- and the cyclic hexamers (SiO3)612-.

The cyclic trimer is found in the mineral benitoite, BaTi(Si3O9.

Chain Polysilicates
Linear (1-D) polymers of formula (SiO3)n2n- can be formed via bridging oxygens. In these structures there is a charge of -2 per silicon atom. A group of minerals called the pyroxene minerals have this type of structure.| enstatite | MgSiO3 |
| diopsite | CaMgSi2O6 |
| spodimene | LiAlSi2O6 |
| pollucite | CsAlSi2O6 |
Linear chains may be linked side-by-side if an oxide ion is replaced with another bridging oxygen atom. If this linking occurs at alternate SiO3 groups in each chain, a double-chain structure (Si4O11)n6n- results. In such structures there is a reduction in the charge and number of oxygen atoms per silicon atom.
Crocidolite, an asbestos mineral of formula Na2Fe5(OH)2[Si4O11]2, is an example. This mineral is fibrous in nature and has fire and heat resistant properties which stems from the long chain structure of the anion.
Sheet Polysilicates
When side-to-side linking of chains is continued indefinitely, more oxides are eliminated and a 2-D polymer results. These 2-D polymers are called sheet silicates and contain the [Si4O10]n4n- anion. Minerals containing this structure are readily cleaved into thin sheets.| micas | muscovite and biotite |
| clay minerals | montmorillonite, kaolinite, china clay and vermiculite |
| talc | |
| soapstone | |
| chrysotile asbestos | |
3-D Polymeric Silicates
Sheets are linked into a 3-D polymer when all the oxide ions are eliminated (all oxygens in the structure are bridging). This structue contains uncharged oxide silica [SiO2]n which is no longer basic but rather an acidic oxide. Many common minerals contain this structure: quartz, flint, jasper, onyx, amethyst, citrine, agate and chalcedony.
Successive polymerization steps:
|

 Place the following minerals in order of increasing degree of polymerization. In order to do this, calculate the O/Si ratio (the lower the ratio the more polymerized the structure.
Place the following minerals in order of increasing degree of polymerization. In order to do this, calculate the O/Si ratio (the lower the ratio the more polymerized the structure.
|
Glass
When acidic silica is reacted with basic oxides at very high temperatures (~1700 0C) and then cooled too rapidly for the polysilicate ions to allow the fomation of the orderly polysilicate ions found in minerals to form. The result is formation of an amorphous solid or glass. Glasses are characterized by having no definite freezing point.Simple glass is made by melting (fusing) sand with sodium bicarbonate and limestone (sources of the basic oxides Na2O and CaO). During this process, silicon-oxygen bridges are broken.
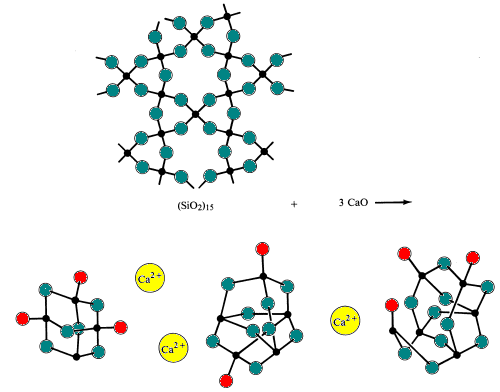
Specialty glasses are made by altering the composition of acidic and basic oxides in the glass.
- Pyrex (tm) glass is unusually resistant to thermal shock. To make it 10-25% B2O3, an acidic oxide, is incorporated into the structure.
- Colored glasses incorporate d-block metal oxides as part of the basic oxide component
- Incorporation of strontium oxide gives a glass that absorbs the x-rays emittd by color TV sets
- The fine optical qualities needed in camera lenses can be obtained by incorporation of La2O3
Learn more about glasses here and at Corning Museum's Glass Resource site.
Soil Chemistry
The fact that increasingly polymerized polysilicate ions have decreasing charges per silicon which results in lowered basicity has importan consequences in soil chemistry.| The more basic the polysilicate anion of a mineral, the more readily it will react with weak acids and undergo weathering. |
Rainwater is somewhat acidic due to dissolved CO2 even in tha absence of sulfur and nitrogen oxides.
Over time rainwater will react with the less polymerixed silicate anions to replace oxide ions with bridging oxygen yielding a more highly polymerized silicate. The oxides are removed as water molecules.
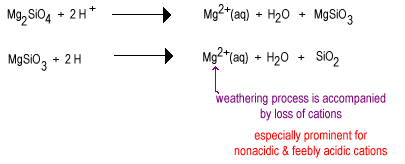
The intermediate stage of weathering has large amounts of layer silicates such as clay as well as some quartz. These soils tend to be found in temperate regions under a cover of grass or trees. Such soils are less fertile than newly irrigated deser soils due to the loss of the nonacidic plant nutrient K+. Layer silicates present in intermediate soils can still hold cations on their negatively charged surfaces which can be released as plant need them. These soils are found in the still quite fertile corn and wheat belts.
Isomorphous Substitution
Polysilicate ions have negative charges that must be counterbalanced by appropriate cations. The terminal oxygens possess negatively charged surfaces that approximate close-packed surfaces of negative charge. The cations that are needed to neutralize the polysilicate's negative charge are located in the layers between the chains or layers or in the tetrahedral or octahedral holes present in the 3-D lattice.The types of cations found in a particular form of polysilicate will depend on"
- the size of the cations
- the charge of the cations
For example olivine, which has an ideal composition of Mg2SiO4, can contain varying percentages of isomorphous subtitution of Fe2+ (radius 92 pm) in place of an equal number of Mg2+ ions (radius 86 pm).
First Principle of Isomorphous Subsititution |
Second Priciple of Isomorphous Substitution |
Isomorphic substitution increases the number of possible substitutions in silicates.
Examples
- K+can be replaced by the rare Rb+ and Tl+ ions as well as the common Ba2+
- Ca2+can be replaced by Sr2+(132 pm), Na+(116 pm), Y3+(104 pm), La3+ (117 pm), and the sixth-period f-block ions (100-117 pm)
- Si4+ can be replaced by the common Al3+ ion (67 pm)
 Which of the following minerals could arise by isomorphous substitution processes in leucite, K(AlSi2O6)?
Which of the following minerals could arise by isomorphous substitution processes in leucite, K(AlSi2O6)?
|