Environmental Applications. Part I.
Common Forms of the Elements in Water
Scenario:
You will study how the variation of pH affects the availability of different elements as nutrients or pollutants in a natural body of water, Purity Springs Lake. Although the chemistry of a natural body of water such as a lake is complex, let's assume that Purity Springs Lake contains no oxidizing, reducing or complexing agents. The only reactions occurring in the lake come from the hydrolysis of hydrated cations.| All hydrolysis processes are equilibria. Most elements will exist in more than one form at any given pH. |
Distribution Diagram
A distribution diagram shows the relative concentrations of the different forms of an element in solution as a function of pH. This type of diagram is generated from experimentally measured concentrations of the species present plotted versus pH.
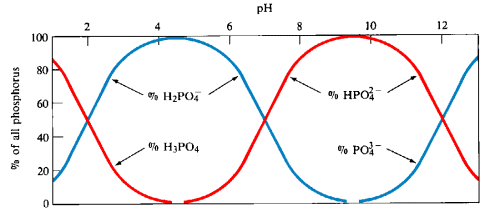
A predominance diagram shows the predominant species that is present in solution at a given pH. This type of diagram can be generated with a minimum amount of calculation using basic concepts about the hydrolysis characteristics of metal cations and oxoanions. You will use predominance diagrams in analyzing Purity Springs Lake.
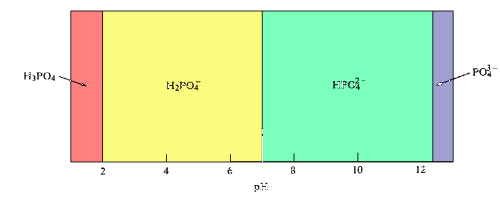
Natural Waters
Most natural waters have pH ranges between 6 and 9.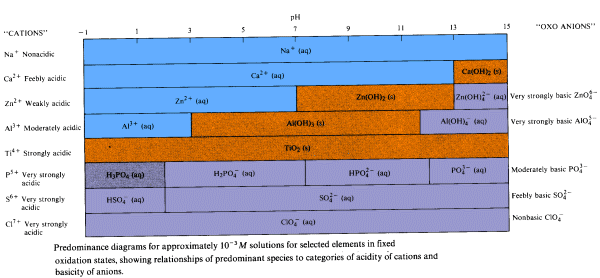
A number of generalizations come from studying predominance diagrams for different elements.
|
in aqueous solution |
|
|
||
|
may be partially hydrolyzed |
|
|
The radioisotopes 99Tc, 90Sr and244Pu (fallout from atomic weapon testing) are deposited
in Purity Springs Lake. The lake has a pH in the range 5.5 to 7. In what predominant form does each
radioisotope exist? Solution: |
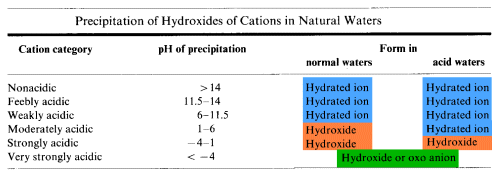
Consequences of Hydrolysis Reactions of the Elements
- Elements having moderately and strongly acidic cations are rarely available for biological activity
- unavailable elements are usually not essential for life or are toxic to life
- exceedingly toxic species such as Pu4+ may be harmful even though most of it is in the sludge at the bottom of the body of water and only a minute amont is present in solution due to equilibrium
- Unavailable elements may become soluble when a body of water becomes highly polluted with acidic or basic pollutants
- acid rain can cause the pH of a poorly buffered lake to drop sufficiently to convert insoluble Al(OH)3 into Al3+ (aq) which is toxic to fish
- raising the pH of an acidic body of water by neutralization may precipitate weakly and moderately acidic ions as hydroxides
- Al3+ (aq)in acidic lake water is converted into gelatinous Al(OH)3 in the more basic environment of fish gills, coating the gills
- fish "sneeze" themselves to death trying to rid themselves of the precipitate
- In acid mine drainage, Fe2(SO4)3 which results from air oxidation of pyrite,dissolves in the water draining from the mine forming sulfuric acid
- acidic mine drainage becomes diluted as it enters uncontaminated streams
- at the higher pH, the hydroxy cation of iron is converted into insoluble Fe(OH)3, an unsightly yellow precipitate called yellow boy
- Elements having weakly or moderately acidic cations which serve as nutrients for plant and animal life may become unavailable in neutral or slightly basic solutions
- Zn2+, Cu2+, Co2+, Fe2+, Mn2+ are weakly acidic essential micronutrients
- these ions precipitate from dilute solutions at pH's in the range 5.3-8.5
- liming acidic soil or lakes with CaO may remove these essential micronutrients
- the moderately acidic Fe3+cation precipitates from dilute solution at pH's above 2.0
- iron-deficiency anemia cannot be treated by ingesting Fe3+ salts because the pH of the intestine where iron absorption occurs is much higher than 2.0