Activity Series of the Metals
Reactivity of Metals with Hydrogen-ion Sources
The activity or electromotive series of metals is a listing of the metals in decreasing order of their reactivity with hydrogen-ion sources such as water and acids. In the reaction with a hydrogen-ion source, the metal is oxidized to a metal ion, and the hydrogen ion is reduced to H2. The ordering of the activity series can be related to the standard reduction potential of a metal cation.| The more positive the standard reduction potential of the cation, the more difficult it is to oxidize the metal to a hydrated metal cation and the later that metal falls in the series. |
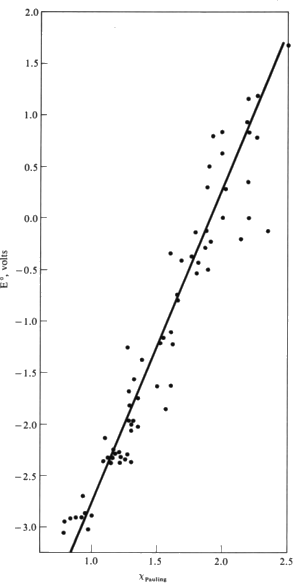
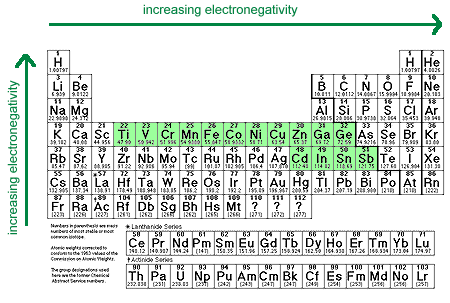
The activity of a metal can be correlated with its Pauling electronegativity.
- Very Electropositive Metals
Most active metals have low electronegativities (EN < 1.4). These cations generally have reduction potentials of -1.6 V or below.

Group Reactivity Features
- react with water to release hydrogen
- goods reducing agents
- not very good oxidizing agents
- ions can't be reduced to the metal in aqueous solution
- Electropositive Metals
Metals in this group have electronegativities that fall between 1.4 and 1.9. Cations of these metals generally have standard reduction potentials between 0.0 and -1.6 V.
Group Reactivity Features
- do not react very readily with water to release hydrogen
- react with H+
- Electronegative Metals
Metals in this group have electronegativities between 1.9 and 2.54. Cations of these metals generally have positive standard reduction potentials.
Group Reactivity Features
- Metals are not oxidized by H+
- These are good oxidizing agents
- Oxidize H2 producingH+ and depositing the metal from an aqueous solution
- Cations of these less active metals will oxidize more active metals to a cation. The less active metal is deposited as the metal.
Other Oxidizing Agents
The activity groups also apply to oxidizing agents such as O2 or oxidizing oxo acids.- Very Electropositive
Metals in this group readily ignite in air (burn) forming the oxides. Fires which result can't be extinguished with water (produces flammable H), CCl4 (an oxidizing agent) or CO2. These fires are best extinguished with sand which smothers the flames and does not react. - Electropositive
These metals do not burn as readily in air. The surfaces of these metals will tarnish in the presence of oxygen forming a protective oxide coating. This coating protects the bulk of the metal against further oxidation (the metal is passivated).
If the surface of the metal is in contact with both oxygen and water, corrosion can occur. In corrosion, the metal acts as an anode and is oxidized.Fe (s)  Fe2+ + 2e-
Fe2+ + 2e-
Some other point on the surface acts as a cathode where oxygen is reduced to OH-. Hydroxide or other ions migrate back to the Fe2+ completing the electrochemical circuit. The ferrous ions precipitate as Fe(OH)2 which further oxidizes and dehydrates to form rust. Corrosion occurs at pH and pE's within the predominance area of Fe2+. Corrosion doesn't occur well in basic solutions due to the coating FeO or Fe(OH)2. - Electronegative Metals
Since many metals in this group are not corroded by oxygen, they are called "nobel metals" and are used in coinage and jewelry. Some in this group are slowly oxidized. The oxides formed are not very stable and can be decomposed by heating.

Metals in this group can be obtained by thermal decomposition of their oxides.
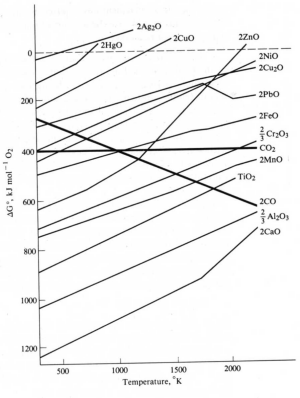
Ellingham Diagram showing the free energies of decomposition of metal oxides as a function of temperature.
Discontinuities result from phase changes of either the metal or the oxides.
Although nonoxidizing acids can't attack electronegative metals, oxidizing acids often dissolve them.
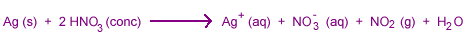
Some electropositive metals do not react with nitric acid because they are passivated.