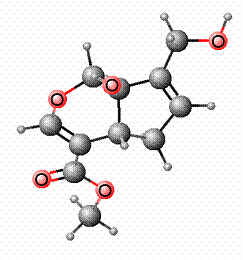
Genipin
Abstract
Genipin is a
hydrolytic product of geniposide, which is found in the fruit of Gardenia
jasminoides Ellis. The components of the
fruit have been used in traditional Chinese medicine and as a blue colorant by
food industries in
General
Information
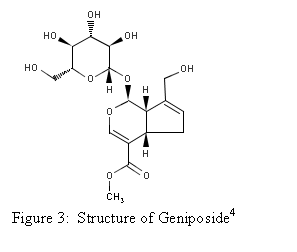
Genipin is an aglycone
derived from geniposide, which is found in the fruit
of Gardenia jasminoides Ellis.
Djerassi and his colleagues discovered the structure of Genipin in the
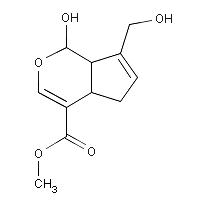
1960’s using NMR spectroscopic data and chemical degradation experiments. It possesses the molecular formula C11H14O5
and contains a dihydropyran ring.1 Genipin itself is colorless but it reacts
spontaneously with amino acids to form blue pigments.2 The blue pigments are edible and are currently
being used as a blue food colorant in East Asia.3 The structure of Genipin is shown in Figure 1
along with other characteristics.
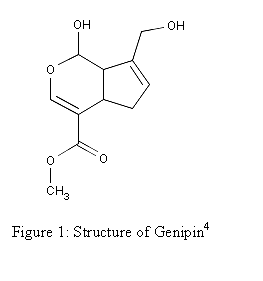 Molecular Formula: C11H14O51
Molecular Formula: C11H14O51
Molecular Weight: 226.227 4
Melting Point: 120-121 °C 2
Systematic Name: Cyclopenta(c)
pyran-4-carboxylic acid, 1,4a-alpha,5,7a-alpha-tetrahydro-1-hydroxy-7-(hydroxymethyl)-,
methyl ester4
Toxicity: Mouse
LD50 intravenous 153mg/kg and Mouse LD50 oral 237mg/kg4
Registry
Number: 6902-77-84
 Natural Source
Natural Source
The fruit of Gardenia
jasminoides Ellis (Figure 2) is the natural plant source of geniposide. It is from the plant family Rubiaceace and is
native to
Isolation of Geniposide and
Genipin
Endo
and Taguchi isolated geniposide and genipin using the following method in
1973. This method is still used however,
some modifications have been made.
Figure 5 shows the reaction.
1.
The Gardenia
jasminoides Ellis fruits are crushed.
2.
The crushed fruit
is then extracted with CHCl3 to remove the fats and oils and then
extracted with MeOH, three times at room temperature.
3.
The combined MeOH
extracts are then concentrated under reduced pressure until it is dry. The result of these steps is a brown colored
mass.
4.
The MeOH extracts
are then chromatographed on charcoal using water and 10% aqueous EtOH to separate
the sugars. Then it is chromatographed
using MeOH to separate the glycosides.
5.
The glycosidic
fraction eluted with MeOH is then rechromatographed on silica gel using a
mixture of MeOH and CHCl3.
Geniposide is eluted with a mixture of 7 to 10% MeOH- CHCl3. The residue is crystallized from acetone and
forms colorless crystals.
6.
Geniposide and a
solution of 1% aqueous solution of β-glucosidase are
added to an acetate buffer (pH 5.0).
7.
This mixture is
allowed to stand at 37°C for three hours.
8.
The reaction
mixture is extracted with ether.
9.
The extract is
concentrated and crystallized with ether-MeOH to yield colorless genipin.7
Geniposide Genipin Figure 4:
Hydrolysis of Geniposide to produce Genipin. Glucose is removed from geniposide.
![]()

β-glucosidase
Genipin and
Biomaterials
Gelatin based materials have recently been studied as a
biomaterial for use in bioadhesives, wound dressing material, and bone
substitute. Gelatin is a natural polymer
which has a low antigenicity and is biodegradable. However, gelatin does possess one important
limitation. It rapidly dissolves in
aqueous environments, this leads to quick degradation at body temperature. One method to improve gelatin is by treating
it with crosslinking materials. Chemical
crosslinking is normally achieved by creating bonds between functional groups
of amino acids. The most popular
chemical crosslinking reagent is glutaraldehyde, a synthetic reagent.8 Recently, reports of the cytotoxicity
of glutaraldehyde due to the release of formaldehyde upon degradation has prompted
research into the development of a natural crosslinking reagent. For this reason, genipin which has low
cytotoxicity and is naturally occurring has been investigated as a new
crosslinking reagent for gelatin.9
Genipin’s Crosslinking
Mechanism
The crosslinking mechanism of genipin with gelatin and
molecules containing primary amines is not well understood at this time and is
still under investigation. Touyama’s
group proposed a mechanism for the reaction of genipin with a
methylamine. His group projected that
the reaction occurred through a nucleophilic attack of the primary amine on the
C3 carbon of genipin. This caused an
opening of the dihydropyran ring. An
attack on the resulting aldehyde group by the secondary amine group then followed.8 This
reaction is shown below.10
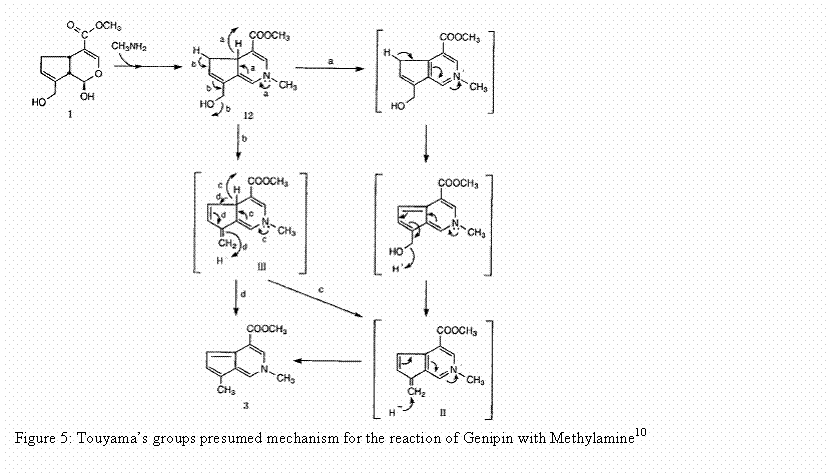
The proposed reaction mechanism with genipin and
methylamine is also believed to be the reaction mechanism for the reaction of
genipin with molecules that contain a primary amine group such as gelatin. The final step in the formation of the
crosslinking material is believed to be dimerization produced by radical
reactions. This indicates that genipin
can be used to form intramolecular and intermolecular crosslinks that have a
heterocyclic structure with materials that contain a primary amine group.11
Bioadhesives and Wound-Dressing
Materials
Bioadhesives are used in surgery for tissue adhesion
and also for stopping the flow of blood.
They are important for sealing air leaks and body fluid links in organs
and tissues that are resistant to suture or stapling techniques. The ideal bioadhesive according to Otani and his colleagues should have reasonable bonding strength
to the tissue, it should be viscous enough that it can be applied to the tissue
area without following to other areas of the tissue, the bioadhesive must also
be flexible enough to adhere to the tissue, and finally the bioadhesive must be
biodegradable and nontoxic.12 The current bioadhesive being used is gelatin
crosslinked with glutaraldehyde.12 However, as stated earlier the toxicity of glutaraldehyde
has led to the investigation of the use of other crosslinking reagents. Sung and his co-workers conducted an
experiment to evaluate gelatin crosslinked with genipin glue to create a bioadhesive.12
They used the gelatin crosslinked with glutaraldehyde glue has a
control for comparison. They compared
the cytotoxicity, the gelation time, bonding strength, and flexibility. They found gelatin crosslinked with genipin glue
to be much less cytotoxic about 10,000 times less then
gelatin crosslinked with glutaraldehyde glue.
The gelation time for the gelatin crosslinked with genipin glue was longer
than that of the gelatin crosslinked with glutaraldehyde glue. The bonding strength of the gelatin
crosslinked with glutaraldehyde glue was greater then the gelatin crosslinked with
genipin glue but still comparable. The gelatin
crosslinked with genipin glue was found to be more flexible than the gelatin
crosslinked with glutaraldehyde glue.
Sung and his co-workers also conducted an in vivo experiment
to test the ability of gelatin crosslinked with genipin glue as a bioadhesive
for closing skin wounds.9 The healing of skin wounds is currently
done by the use of sutures but sutures increase the risk of infection and require
the use of local or general anesthesia. The
in vivo test compared the use of sutures, gelatin crosslinked with glutaraldehyde
glue, and gelatin crosslinked with genipin glue. They found that the tensile strength of the
skin across the wounds increased with time for both the glues and the suture. All three techniques did not interfere with
the healing of the wound and no calcification was seen. It took six days for the suture to be reabsorbed
while it took nine days for the gelatin crosslinked with genipin glue to be reabsorbed. The gelatin crosslinked with glutaraldehyde
glue took fourteen days to be completely reabsorbed. The healing process of the suture was more
rapid then the glues. The wounds treated
with gelatin crosslinked with genipin glue healed faster and less inflammation was
seen then in the wound treated with gelatin crosslinked with glutaraldehyde glue. Both of these experiments,
indicate that further investigation into the use of gelatin crosslinked with genipin
glue as a bioadhesive is warranted.
The results of the previous two studies encouraged
Chang and his colleagues to develop a genipin crosslinked gelatin membrane to use
as a wound dressing material.11 They used a glutaraldehyde crosslinked gelatin
membrane as the control. Their in vitro study
indicated that the degree of crosslinking for both was equivalent. The tensile strength of the genipin
crosslinked gelatin membrane was much greater than the glutaraldehyde crosslinked
gelatin membrane; however, the strain at fracture was much less in the genipin
crosslinked gelatin membrane than the glutaraldehyde crosslinked membrane. The swelling ratio of the genipin crosslinked
membrane was smaller then the swelling ratio for the glutaraldehyde crosslinked
gelatin membrane. The genipin
crosslinked membrane degraded at a slower rate then the glutaraldehyde crosslinked
gelatin membrane when exposed to collagenase solution. This can be attributed to the higher stereohindrance of the cyclic genipin crosslinked gelatin membrane. In the in vivo study, they found that the
inflammation in the genipin crosslinked gelatin membrane was less severe
throughout the course of the experiment which was 21 days. This indicates the genipin crosslinked gelatin
membrane has a better biocompatibility then the genipin crosslinked gelatin membrane. The healing rate of the wound using the
genipin crosslinked gelatin membrane was also much faster then the healing time
of the wound for the genipin crosslinked gelatin membrane.
Bone Substitute
The ability to repair bone defects still presents many
challenges. Bone defects can be various
sizes and shapes caused by trauma, tumors, or infections. In bone transplantation, bone from somewhere
else in the body can be used but this has the disadvantage of limited bone
supply and donor site morbidity. Bone
can also be transplanted form another individual but this can cause an immune
response and can transmit diseases. Therefore,
synthetic bone-promoting materials are needed to repair bone defects. The ideal bone composite material would
possess properties such as good biocompatibility, good biodegradation, and be
able to get cells into the defect site and promote new bone formation.13
Tricalcium phosphate has been
investigated as bone substitute. However,
because it is granular it is hard to shape to the defect site on the bone. Therefore, studies and investigations have
been conducted using material composed of genipin crosslinked gelatin and tricalcium
phosphate.13 Yao and his
co-workers evaluated the use of a genipin crosslinked gelatin mixed with tricalcium
phosphate bone substitute in an in vivo study using rabbit calvarial bone.13 In this study they found that the mixture was
biocompatible; there was no inflammation found in the tissue below the mixture. The mixture was also malleable and was easy
to mold to the shape and size of the defect. It also was biodegradable and aided in the growth
of new bone through the release of gelatin and Ca2+. The study also indicated that growth of new
bone was occurring in the defected area and that the mixture of tricalcium phosphate
and genipin crosslinked gelatin was being replaced by the new formed bone. Their study indicates the genipin crosslinked
gelatin mixed with tricalcium phosphate can be valuable as a bone substitute
when repairing bone defects.
Genipin as a
Fingerprint reagent
Genipin is also being investigated in the field of forensic
science as a fingerprint reagent to develop latent fingerprints on paper
products. In an article by Lee and his collogues,
they investigated the use of genipin as a way to development amino acid stains.14 They
compared genipin’s ability to develop amino acid stains with ninhydrin’s
ability. The product of the reaction of
genipin with amino acids was very stable and lasted 7 months, but the ninhydrin
product disappeared much sooner than the genipin product. The sensitivity was also greater with genipin
then ninhydrin as seen through the molar absorptivities. Because of the properties genipin showed in
this study, Almog and collogues investigated genipin as a fingerprint reagent.15 Ninhydrin
is the current reagent used to develop latent fingerprints on paper products. Almog and co-workers define an ideal reagent
for developing latent fingerprints as a reagent that produces color and also fluorescent. It must also be a simple reaction that can be
done easily at a crime scene. Lastly, it
must be highly sensitive. They found
that the fingerprints developed with genipin were blue in color and showed
clear ridge detail. They were also able
to visualize weak fingerprints using fluorescence and genipin shifted the excitation-emission
domain toward the longer wavelength which produces less background. They concluded that genipin meets the
requirements for a fingerprint reagent and should be considered as an alternate
to ninhydrin. In a second study, Almog
and co-workers showed the advantages of genipin over ninhydrin.16 Genipin has the advantage of the combination
of both color and fluorescence in a single reaction and as stated early it has
a low background when fluoresced because it is viewed at longer wavelengths. Genipin is also safe and environment friendly
because it is a natural plant product. The
safety of genipin is very important because ninhydrin has recently been
reported to potentially cause health hazards.
Besides the cost of genipin they concluded that it has great potential
to be used as a fingerprint reagent.
Conclusion
Genipin is a naturally occurring
material that shows great potential as a use in biomaterials and also as a
fingerprint reagent. As stated earlier
genipin crosslinked material has been use as a bioadhesive, in wound dressing
materials, and as a bone substitute. In
addition to these studies, studies have also been conducted to investigate
genipin crosslinked gelatin as a conduit for peripheral nerve regeneration17
and genipin crosslinked chitosan for protein drug delivery18. Based on these novel experiments, I believe that
genipin will become an important crosslinking reagent for biomaterials in the future. I also believe it can be valuable as a
reagent to develop latent fingerprints.
References
1. Djerassi
C., Nakano T., James A.N., Zalkow L.H., Eisenbraun E.J., Shoolery J.N. Terpenoids. XLVII. The Structure of Genipin. J. Org. Chem. 1961, 26, 1192-1206.
2. Djerassi
C., Gray J.D., Kincl F.A. Naturally Occurring Oxygen Heterocyclics. IX. Isolation and Characterization of
Genipin. J. Org. Chem. 1960, 25, 2174-2177.
3. Park J.E., Lee J.Y., Kim
H.G., Hahn T.R., Paik Y.S. Isolation and Characterization of Water-Soluble
Intermediates of Blue Pigments Transformed from Geniposide of Gardenia
Jasminoides. J. Agric. Food Chem. 2002, 50, 6511-6514.
4. Natural Library of
Medicine. Specialized Information Services. ChemIDplus Advanced. Full Record Genipin.
http://chem.sis.nlm.nih.gov/chemidplus/jsp/common/ChemFull.jsp?MW=226.227
(accessed
5. Tsai T.R.,
Tseng T.Y., Chen C.F., Tsai T.H. Identification and Determination of Geniposide
Contained in Gardenia Jasminoides and in Two Preparations of Mixed Traditional
Chinese Medicines. J. Chromatogr. A.
2002, 961, 83-88.
6. Ishikawa
S. Picture Book of Jia-Wei-Gui-Pi-Tang. http://web1.incl.ne.jp/ishikawa/PET/pict.html
(accessed
7. Endo T., Taguchi H. The Constituents of Gardenia Jasminoides Geniposide and Genipin-getiobioside. Chem.
Pharm. Bull. 1973,
21, 2684-2688.
8.
9. Sung H.W., Huang D.M.,
Chang W.H, Huang L.L.H., Tsai C.C., Liang
I.L. Gelatin-derived Bioadhesives for Closing Skin Wounds: An in vivo Study. J. Biomater. Sci. Polymer Edn. 1999,
10, 751-771.
10. Touyama
R., Inoue K., Takeda Y., Yatsuzuka M., Ikumoto T., Moritome N., Shingu T., Yokoi T., Intuye
H. Studies on the Blue Pigments Produced from Genipin and Methylamine. II. ON
the Formation Mechanisms of Brownish-Red Intermediates Leading to the Blue
Pigment Formation. Chem. Pharm. Bull. 1994, 42, 1571-1578.
11. Chang W.H., Chang Y., Lai
P.H., Sung H.W. A Genipin-Crosslinked Gelatin Membrane as Wound-Dressing
Material: in vitro and in vivo Studies. J.
Biomater. Sci. Polymer Edn. 2003, 14, 481-495.
12. Sung H.W., Huang D.M.,
Chang W.H., Huang R.N., Hsu J.C. Evaluation of Gelatin
Hydrogel Crosslinked with Various Crosslinking Agents
as Bioadhesives: In vitro Study. J. Biomed. Mater. Res. 1999, 46, 520.
13.Yao C.H., Lui B.S., Hsu S.H., Chen Y.S. Cavarial
Bone Response to Tricalcium Phosphate-Genipin Crosslinked Gelatin Composite. Biomaterials 2005, 26, 3065-3074.
14. Lee S.W., Lim J.M., Bhoo S.H., Paik Y.S., Hahn T.R. Colorimetric Determination
of Amino Acids Using Genipin from Gardenia jasminoides. Analytica Chimica Acta 2003, 480, 267-274.
15. Almog J., Cohen Y., Azoury M., Hahn T.R. Genipin-A Novel Fingerprint Reagent
with Colorimetric and Fluorogenic Activity. J.
Forensic Sci. 2004, 49, 255-257.
16. Levinton-Shamuilov
G., Cohen Y., Azoury M., Chaikovsky
A., Almog J Genipin, a Novel Fingerprint reagent with Colorimetric and Fluorogenic
Activity, Part II: Optimization, Scope, and Limitations. J. Forensic Sci. 2005, 50, 1367-1371.
17. Chen, Chang, Cheng, Tsai,
Yoa, Lui An in vivo Evaluation
of a Biodegradable Genipin-Cross-Linked Gelatin Peripheral Nerve Guide Conduit
Material. Biomaterials 2005, 26, 3911-3918.
18. Chen, Wu, Mi, Lin, Yu, Sung
A Novel pH-Sensitive Hydrogel Composed of N,O-carboxymethyl Chitosan and
Alginate Cross-linked by Genipin for Protein Drug Delivery. J. Controlled Release 2004, 96, 285-300.
BY: BRENDA VAANDERING