|
|||||||||||||||||||
|  | ||||||||||||||||||
 | |||||||||||||||||||
 | |||||||||||||||||||
 | |||||||||||||||||||
 | |||||||||||||||||||
|  | ||||||||||||||||||
| 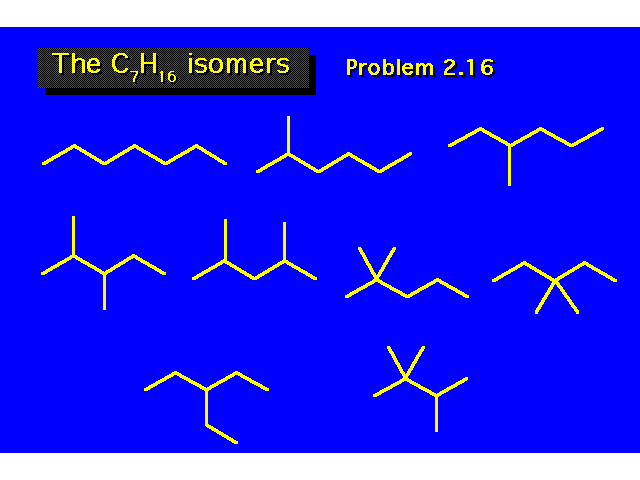 | ||||||||||||||||||
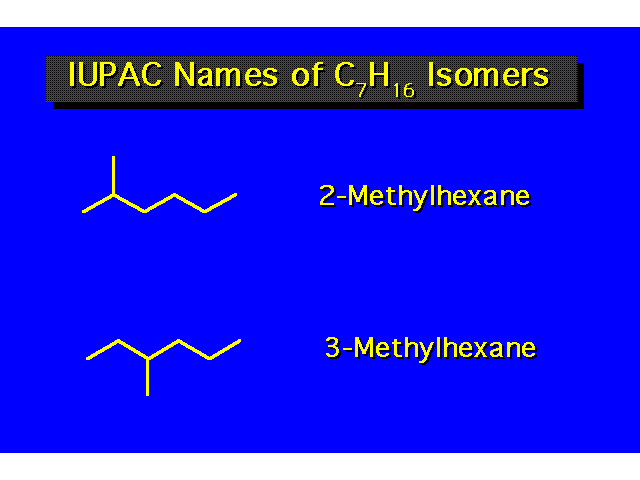 | |||||||||||||||||||
| When there are two or more different types of alkyl groups branching off the main chain, locate them each by number and list them alphabetically. When the same branching group appears more than once in a structure, the number times the group appears is indicated by a prefix:
The position of each group is listed separately by a locant number. The locant numbers are separated from each other by commas. The prefixes are disregarded in alphabetizing. | 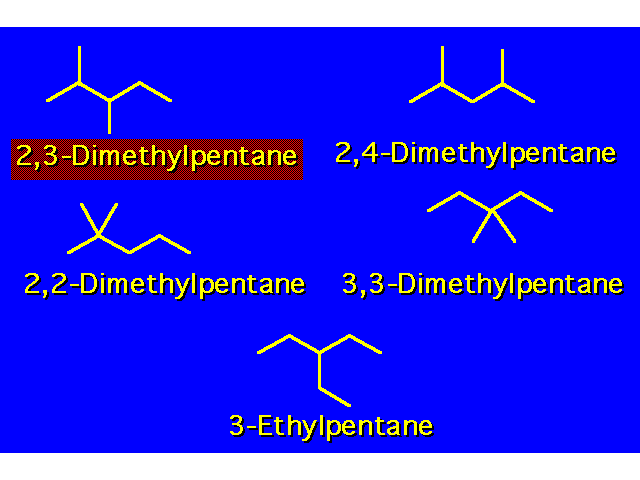 | ||||||||||||||||||
 | |||||||||||||||||||
| In complicated structures the "first point of difference" rule is useful in determining the proper way to number the parent chain. When numbering this molecule the first branch occurs at position 2 from either direction. In numbering from the right, the second branch is also located at position 2. Numbering from the left, the second group would be at position 3. This is the first point of difference, and the group is given the lower number. | 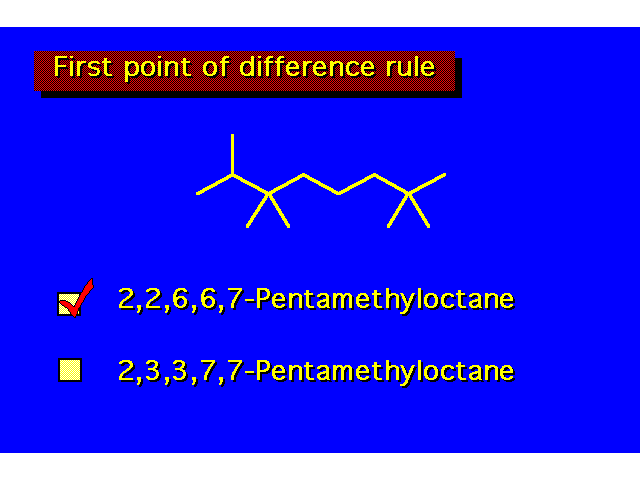 | ||||||||||||||||||
 | |||||||||||||||||||
 | |||||||||||||||||||
 | |||||||||||||||||||
 | |||||||||||||||||||
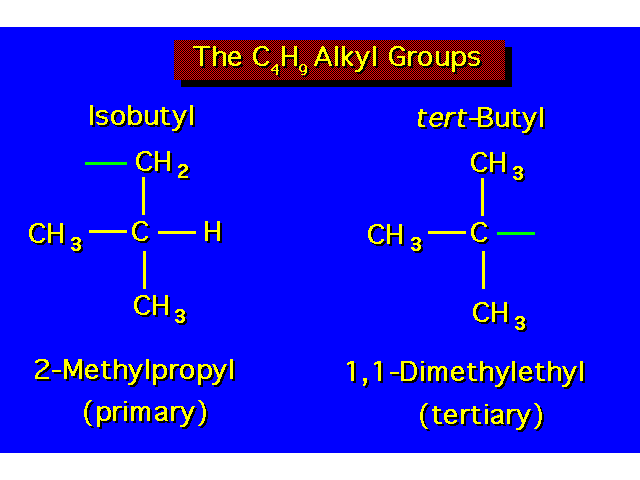 | |||||||||||||||||||
|  | ||||||||||||||||||
For cycloalkanes, the prefix cyclo- is added to the name of the corresponding open chain alkane. Substituent positions are indicated by a number. Always begin numbering from a carbon bearing a substituent. Number around the ring giving the substituents the lowest possible numbers. | 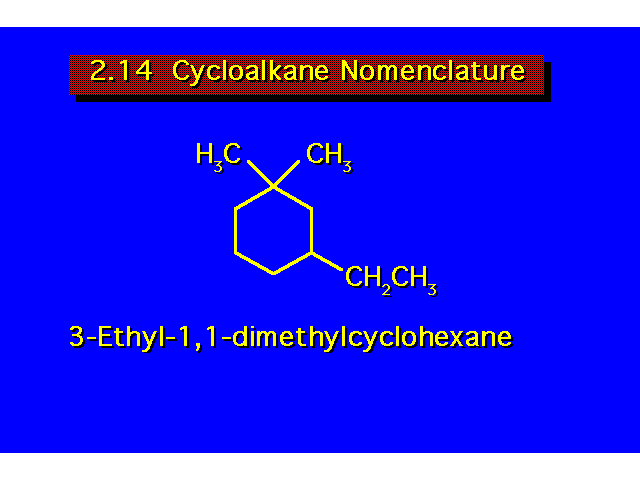 | ||||||||||||||||||
| |||||||||||||||||||
 Name the following compounds: Name the following compounds: | |||||||||||||||||||
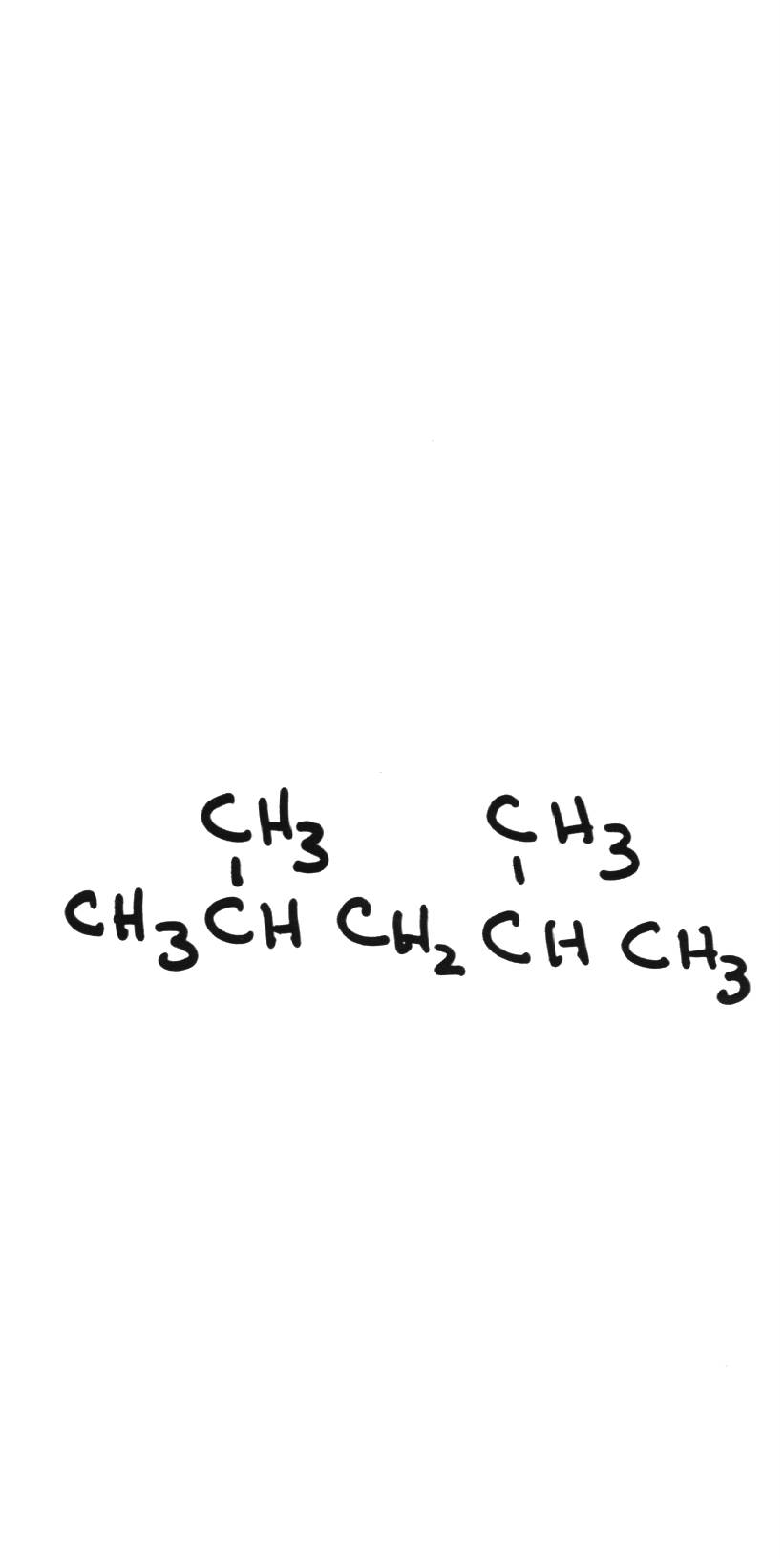 | 2,4-dimethylpentane | ||||||||||||||||||
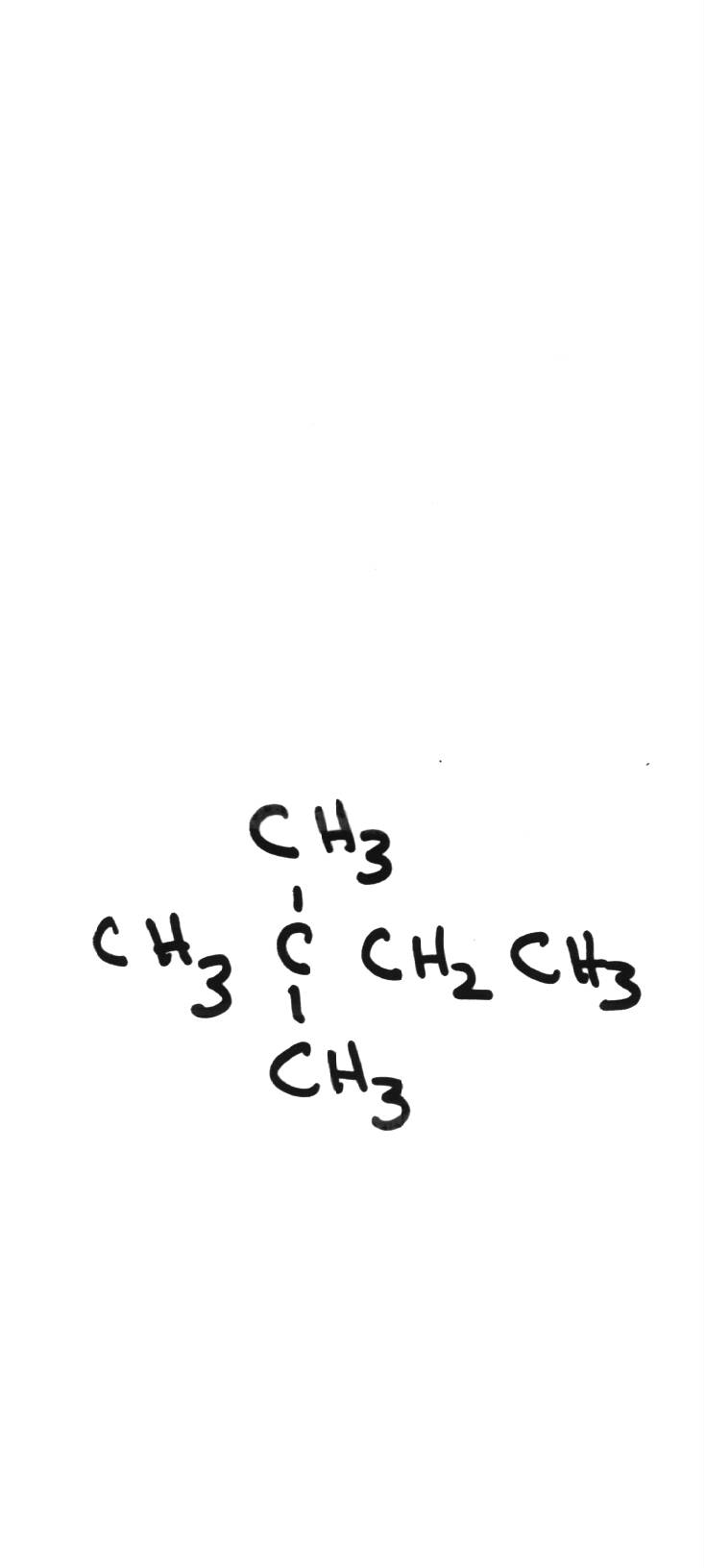 | 2,2-methylbutane | ||||||||||||||||||
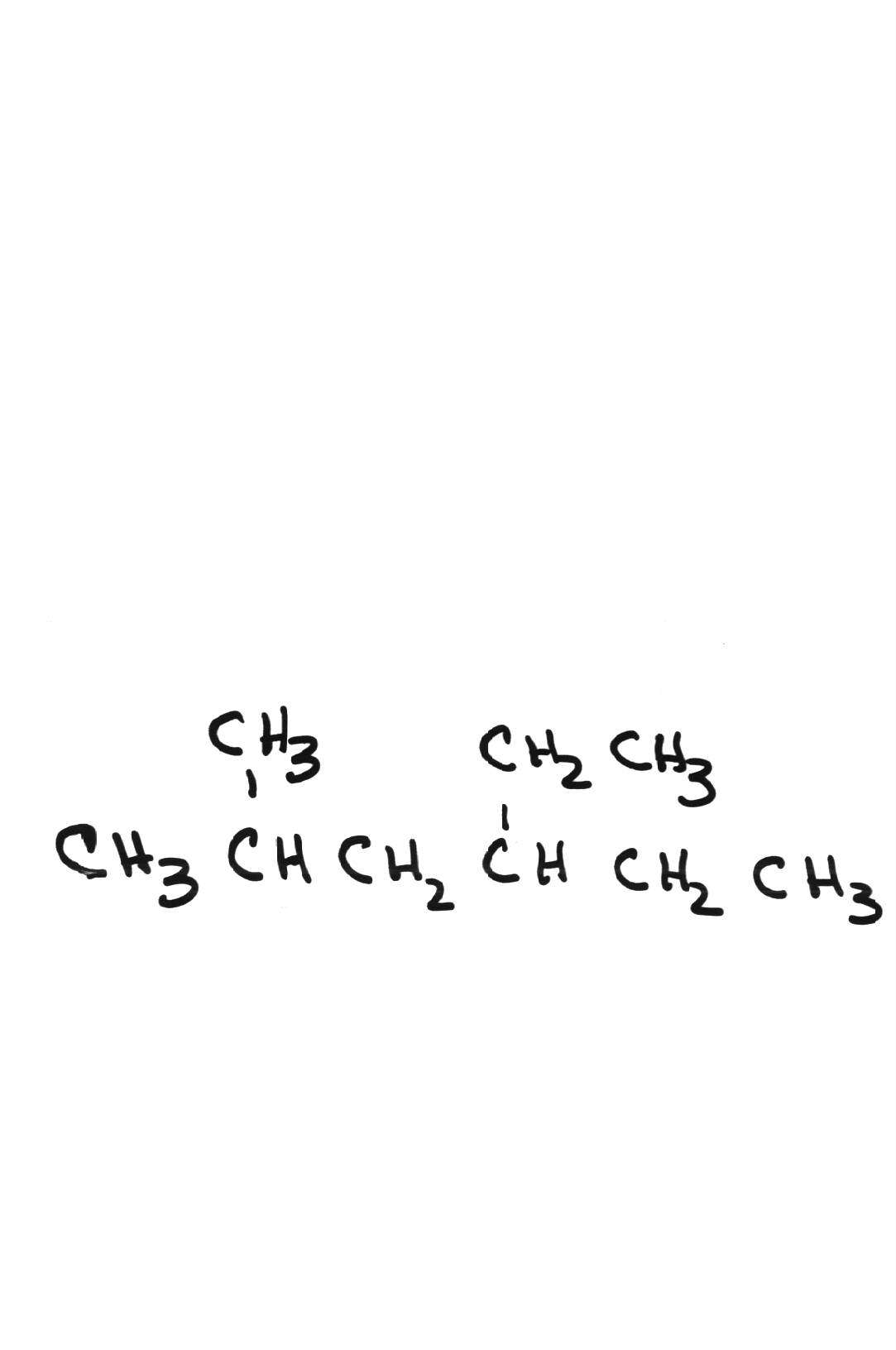 | 4-ethyl-2-methylhexane | ||||||||||||||||||
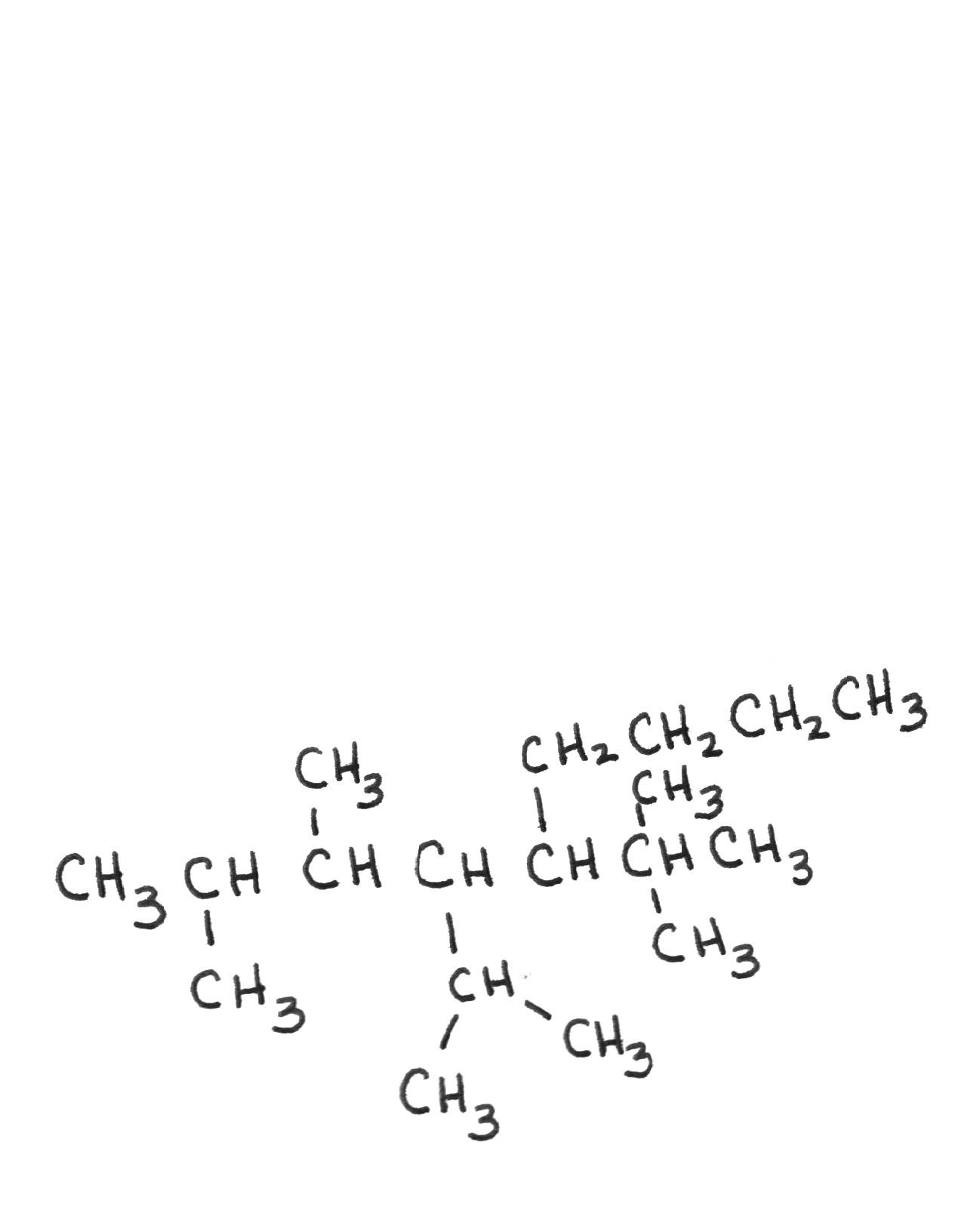 | 5-tert-butyl-4-isopropyl-2,3-dimethylnonane | ||||||||||||||||||
