
Author: Aaron Vorderstrasse
School: Western Oregon University
Location: Monmouth, Oregon
Abstract: Dichlorodifluoromethane is an inert gas that has a long history acting as a refrigerant, allowing us to stay cool in the summer, and as a spray propellant for important consumer substances. Dichlorodifluoromethane owes many of its desirable properties to its C-F bonds and synthesis involves organofluorine chemistry. Despite many uses, the gas has been isolated as a major contributor to ozone depletion and it is currently banned for production in the United States. Dichlorodifluoromethane use in the United States is currently regulated and substitutes for dichlorodifluoromethane are promising, although they too have their drawbacks.
Identification:
Synonyms14: dichlorodifluoromethane; algofrene type 2; arcton 12; arton 6; carbon dichloride difluoride; CF 12; CF 12(halocarbon) CFC 12; CFC-12; chladone 12; dichlorodifluoromethane (CCL2F2); dichlorodifluoromtheane (DOT French); dichlorodifluoromethane (DOT); diclorodifluorometano (DOT Spanish); difluorodichloromethane; dymel 12; electro-CF 12; eskimon 12; F 12; F 12 (halocarbon); F-12; FC 12; FCC 12; FWK 12; fluorocarbon 12; forane 12; freon 12; freon F-12; freon ® 12; frigen 12; frigen R12; fron 12; gas refrigerante R-12 (DOT Spanish); gaz refrigerant R-12 (DOT French); genetron 12; genetron ® 12; halon 122; halon ® 122; HC 12; isceon 122; isotron 12; khladon 12; ledon 12; methane, dichlorodifluoro-; propellant 12; R 12; R 12 (refrigerant); refrigerant 12; refrigerant gas R-12; refrigerant R 12; SDD 100; and ucon 12.
Regulatory Name: CFC-12, Dichlorodifluoromethane
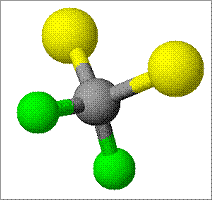 Formula: CC12F2
Formula: CC12F2
DOT Label: Non-flammable Gas
CAS: 75-71-8
STCC: 4904516, 4904561
CHRIS: DCF
UN Number: 1028
Structure: (Black = Carbon, Yellow = Fluorine, Green = Chlorine)
Physical Properties: Colorless gas with a characteristic ether-like odor at >20% by volume.
MW: 120.914 g/mol
BP:
-29.8°C
VP:
5.7 atm
MP:
-158°C
ASHRAE Naming [17,21]: Refrigerating
compounds are often referred to by their standard refrigerant designation
codes, proposed by The American Society of Heating and Refrigeration
Engineering in 1957 [21], prefixed by CFC for chlorofluorocarbon or R for refrigerant. The code is three digits with the number of
fluorine atoms in the ones place, the number of hydrogen atoms plus one in the
tens place and the number of carbon atoms minus one in the hundred’s place. Thus, dichlorodifluoromethane in ASHRAE is R-012
or CFC-012, but the zero is usually dropped to give CFC-12.
History: In the world of 2005, if we need
a cold drink we might go to the refrigerator for a few ice cubes or if there is
a fancy model of refrigerator available, then we might have ice water right on
tap! Things weren’t always like this
however, that is before modern refrigeration.
In the world of 1810 in Cuba, the ice for our iced drink would need to be imported from the New England states at more than 500 dollars per the ton10 – that’s a lot of 1810 money! Obviously ice is a very important thing if Boston, at the same time, exported approximately 65,000 tons10 of ice per year; this is before mechanical refrigeration. Ice traditionally has been very important not only in good drinks, but it has also been critical to hospitals. It is then appropriate that a doctor, Scottish Dr. John Gorrie, received the first patent for mechanical refrigeration in 1842 to help his feverish patients1.
After the advent of mechanical refrigeration, the need for ice shipped from temperate climates began to drop10. By 1855 the man made ice was being used in breweries and meat plants10, but the new ice machines weren’t without problems. First, the refrigerant of choice for the 19th century ice machine is ammonia, which has the drawbacks of being highly toxic, corrosive, and difficult to compress [1,8,10]. The net result is that the ice machines were massive (as big as a typical kitchen), steam powered (the best source of energy in the 19th century for large equipment – needing constant boiler attendance), required a lot of maintenance and were the source of industrial accidents10. An alternative had to be found!
Chemists, on the job, made a technological breakthrough: Sulfur dioxide is compressed readily and has a good latent heat* of 25 kJ/mol [8]! Chemists and physicists were able to put a kitchen sized version of the refrigerator on the market after World War One [10]. Unfortunately, sulfur dioxide isn’t the most pleasant refrigerant: Early refrigerators leaked and if they didn’t, sulfur dioxide is corrosive, so they soon would [8]. Additionally, sulfur dioxide is noted for its odor [10].
These early refrigerants were just not satisfying the public: they wanted something that would not stink up the house, burn it down, or kill them outright! It is with this criterion in mind that Frigidaire Division of GM set out to come up with a solution. They appointed Robert McNary, Thomas Midgley and Albert Henne [10] to the task of finding performing, inert refrigerants for use in the household. It is this team that discovered dichlorodifluoromethane as a refrigerant in 1928 [21].
*Latent heat is described as “a change of state without a change in temperature” [2] or basically the amount of energy that a refrigerant can absorb per refrigeration cycle through changing back and forth between the liquid and gas state.
Why are some Refrigerants better than others?
Toxicity: Up until the discovery of Freon, Dupont’s trade name for dichlorodifluoromethane, the only available “classical” refrigerants NH3, SO2 and CH3Cl all had great drawbacks and Freon was a welcomed alternative [10]. One of the primary goals in new refrigerant research at the time was that the new refrigerant be non-toxic. Chlorofluorocarbons decrease in toxicity and, very desirably, decrease in boiling point with increased C-F bonding [5]. With two C-F bonds Freon is non-toxic and boils around -29.8°C [2]. The toxicity of Freon is perhaps best demonstrated when Thomas Midgley Jr., the discoverer of CFC-12, famously filled his lungs with Freon and extinguished a candle at a meeting of the American Chemical Society in1930 [8]. The only real danger occurs if Freon is exposed to flame, according to Dupont’s Freon MSDS, the gas may decompose into its parts (HF and HCl) and, according to Scott Specialty Gases’ Freon MSDS [18], the gas may decompose into the chemical warfare agent phosgene CCl2O [2,18].
Desirable Properties: When the Midgley team was looking for refrigerants efficiency was high on their list of desirable properties; it takes less power to run a refrigeration compressor if the refrigerant is easily compressible. Thus, vapor pressure and boiling point became paramount. For a temperature of about 40°C inside the refrigerator, the refrigerant needs to boil below -10°C [8] and above -60°C [5]. The refrigerant should also be inert, for safety, and low cost so that customers can afford systems. With this in mind, here is an example from Dr. William Gumprecht’s Refrigerants for the 21st Century online class at Kennesaw State University [8]:
CO2 seems like it would be a great refrigerant: it’s low cost, non-toxic, non-flammable and doesn’t react with much. The big problem, however, is that at -25°C CO2 has a vapor pressure of 15 atm; this is the temperature at which we want to expand the gas to absorb heat. An even bigger problem is that at 50°C CO2 has a vapor pressure of greater than 60 atm; this could simply blow the refrigerator apart! See the following table of refrigerants:
|
|
MW |
bp°C / |
Latent Heat |
Toxic? |
Pressure at 0°C |
Pressure at 50°C |
|
NH3 |
17 |
-34 |
24kJ/mole |
Yes |
4atm |
20atm |
|
CO2 |
44 |
-78 |
25kJ/mole |
No |
35atm |
>>60atm |
|
SO2 |
64 |
-10 |
25kJ/mole |
Yes |
2atm |
9atm |
|
CF2Cl2 |
121 |
-30 |
22kJ/mole |
No |
3atm |
12atm |
Remember, the target refrigerant boils from -60 / -10°C, with low vapor pressure at low temperature, and slightly elevated vapor pressure at higher temperature. Additional requirements are that the gas is inert and can carry away a large amount of heat (referred to as latent heat).
With the above characteristics in mind, and with the knowledge that Freon is inert, it appears that dichlorodifluoromethane is an ideal refrigerant for the home refrigerator. The only area in which Freon is weak is the amount of heat that it can carry away per refrigeration cycle; this is exactly what the Frigidaire team found.
Synthesis of Dichlorodifluoromethane and Organofluorine Chemistry:
Belgian chemist Fredric Swarts had already found an effective fluorine exchange catalyst SbF3Br2 in the 1890’s [8,21]. With a little reaction manipulation, the Frigidaire team was using it to make Freon on the lab scale.
The basic lab synthesis of dichlorodifluoromethane that Midgely and his team discovered is as follows [8]:
(1) CCl4 + HF –SbF3Cl2 (catalyst) → CFCl3 + CF2Cl2 (Freon-12) + HCl
Dr. Michael Dove at the University of Nottingham in the UK found in 1976 that HF was actually in equilibrium with SbCl5 [8]. Apparently, fluorine is exchanged for chlorine and SbCl5 becomes SbFxCl5-x but, the addition of the last fluorine, to give SbF5, is very hard to accomplish8. Additionally, it was found that each fluoride in SbFx could be exchanged for a chlorine atom in chlorocarbons8. The most effective fluorinating agent is SbF5 and the least effective is SbFCl4; temperature also adds to effectiveness8. Companies were able to take advantage of this in industrial synthesizes.
Industrial
synthesis of Freon requires the process to be economical and smooth
flowing. According to a patent, for
production of organic fluorine compounds, issued to Kinetic Chemicals [5],
Freon can be produced by the following reaction:
(2) HF(g) + CCl4(g) –Carbon catalyst→ CCl3F + CCl2F2 + CClF3
Products are dependent on reaction conditions. This process is “smooth and continuous and proceeds without undesirable side reactions.” [5] The products then undergo a complicated scrubbing process to purify them.
Chlorofluorocarbon Uses:
CFC production, including Freon production, peaked in the mid-1980s [8,15]. The main applications of CFC’s have been use as refrigerant gases, propellants and blowing agents among others [15,21].
Refrigeration: We’ve all experienced air conditioning before; an ice cold room on an 85° day! Air conditioning is just refrigeration for a large space. As a refrigerant, R-12 is desirable because of its many exceptional properties, which we’ve already discussed, but how does refrigeration keep us so cool on that hot day?
Refrigeration is nothing more than
the transfer of heat, Qc, from a cooler area to a
warmer area. This idea is counter-intuitive because we generally think of
heat transfer from hot to cold, but the idea works because mechanical pumping
energy is required to proceed. 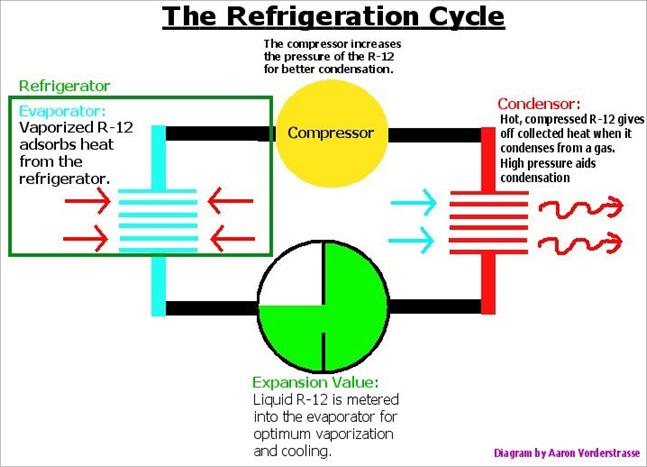
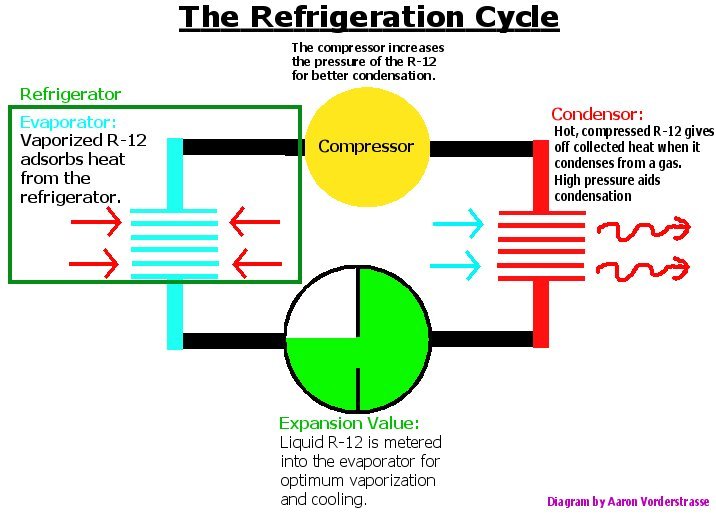
The basic principle behind mechanical refrigeration is the pumping of a low boiling “refrigerant” around a closed circuit system. On its journey through the refrigeration loop the liquid refrigerant is evaporated in coils, inside the cold cabinet, and heat energy, Qc, from the refrigerator is absorbed. It is through this process that the cabinet is cooled. The vapor is then compressed, giving an elevated boiling point, and allowed to condense in a set of coils outside the cabinet. It is in these coils that the refrigerant then gives up the absorbed heat Qc to the environment. The process is called the Clausius-Rankine cycle [20].
The coefficient of performance for the Clausius-Rankine cycle is given by abs(Qc/W) or the amount of energy removed, divided by the amount of work it took to remove that energy [20]. Thus, the amount of heat which can be removed per refrigeration cycle is important, which pertains to the latent heat of a given refrigerant (68 BTU / pound for Freon) [2].
We see refrigeration systems utilizing R-12 all the time: If a car is built before 1994, and is equipped with air conditioning, chances are that it’s charged with R-12. However, R-12 use is prohibited in new vehicles after 1994 in accordance with the Montreal protocol.
Blowing and Propelling: R-12 or CFC-12 makes a desirable blowing and propelling agent because it is non-reactive, non-toxic and a gas at room temperature. As a blowing agent, before the 1990 clean air act, CFC 12 was used on the space shuttle to blow in foam insulation in important parts of the shuttle where foam structure is critical [4]. As an aerosol propellant CFC 12 can be used in several important consumer applications21. To treat jungle fever, CFC 12 was produced as a canned aerosol, and the customer could simply spray themselves down with boiling Freon to keep cool! Of course, too much Freon in one spot may cause frostbite [2].
Environmental Problems:
Dichlorodifluoromethane, that we’ve all come to depend on, unfortunately is associated with some gigantic environmental problems. One of the main selling points of Freon in the 1930’s was that it didn’t kill people because it was inert. The reason Freon is inert is because its C-F bonds are hard to break and thus very stable. Freon is so stable in fact, that it cannot be removed from the atmosphere by earth’s normal processing system involving rain and hydroxyl radicals [3,13,17]. CFC-12 has a life of 102 years [6,21] and that lifespan gives the gas plenty of time to make it into the upper stratosphere where it is decomposed by solar radiation. After CFC-12 decomposes, free chlorine radical can destroy ozone. Ozone is responsible for stopping harmful ultraviolet radiation from the sun, the kind that causes skin cancer, yet it is only present in small amounts, 3 parts in 107 parts atmosphere are ozone [17], in the stratosphere.
Ozone is naturally produced and destroyed in the atmosphere at high altitude ~30 km by reaction of oxygen and solar radiation though the Chapman reactions21:
Natural Ozone Production [17, 21]:
(3) O2 + hν → O + O ; λ < 242 nm (UV-C) in the upper atmosphere.
(4) O2 + O + M → O3 + M ; M is a neutral atom like oxygen or nitrogen.
Natural Ozone Destruction 17, 21:
(5) O3 + hν → O + O2 ; λ = 240-320 nm (UV-B)
(6) O3
+ O → 2O2
In 1971 Crutzen [3] provided additional natural reactions involving nitrogen [3,17,21]:
(7) NO + O3 → NO2 + O2
(8) NO2 + O → NO + O2
Now, the artificial reactions that occur as a result of CFC release: When a solar photon strikes a chlorofluorocarbon, such as CFC-12, chlorine can be released to catalytically destroy ozone [12,13,21].
Chlorine acts as a catalyst to destroy ozone by the following process [3,12,13,17]:
(9) CCl2F2 + hν (λ < 220 nm) → Cl + CClF2 [17, 21]
(10) O3 + hν → O + O2
(11) O3
+ Cl → ClO + O2
(12) O + ClO
→ Cl + O2
(13) Net: O + O3 → 2O2
These reactions were put forth by Molina and Rowland in 1974 [12]. Note that this process requires free oxygen atoms to proceed. It was originally believed by Molina and Rowland that ozone depletion by chlorine radical could only occur in the upper atmosphere where there is plenty of free oxygen present13.
Also put forth are terminating reactions which form temporary reservoirs. The most important terminating reactions are those involving HCl, ClNO3 and HOCl [3,13]:
(14) Cl + CH4 → HCl + CH3 [9]
(15) ClO + NO2 + M → ClONO2 + M
(16) ClO + HO2 → HOCl + O2
However, these reservoirs are only temporary and the radicals can be regenerated by the following reactions [13]:
(17) HCl + OH → Cl + H2O
(18) ClONO2 + hν → Cl + NO3
(19) HOCl + hν → Cl + OH
Because of these reactions, chlorine radical only exists in the form necessary to destroy ozone for a small portion of time, but it still seems to get the job done.
Polar Ozone Loss: 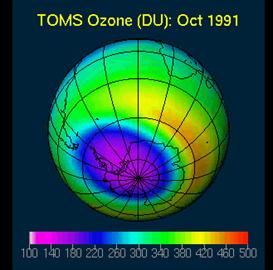
When we hear someone talking about ozone loss, most people probably think about the Antarctic ozone hole discovered by Fahmar et al. in 1985. The freely distributed picture at right is from Cambridge and depicts the ozone hole over Antarctic in 1991. 1 Dobson (DU) = 10-3 atm*mm ≈ 2.69 x 1016 molecules*cm-3 at STP [21].
Molina and Rowland predicted [13] that catalytic ozone destruction near the poles would decrease due to a decrease in solar radiation at the poles. The sun’s rays strike the poles indirectly, so not much radiation is available to break down the reservoir species formed in 14-16. Additionally, they thought that the decreased temperature would slow reactions 17 and 18.
Fahmar suggested a possible link to stratospheric chlorine concentrations and the decomposition of CFC’s. Somehow ozone over Antarctica was being devastated!
There was a buzz in the scientific community and several different theories about ozone depletion were put forth. It was known that polar stratospheric clouds (PSC), made mainly from HNO3·3H2O [13, 21,22] or ice [22], float over Antarctica. With nitrogen devoted to polar clouds, it is unavailable for use in radical terminating reaction 18. Chlorine is activated on PSCs by the following primary reactions [7,13,17,22]:
(20) ClONO2 + HCl → Cl2 + HNO3
(21) ClONO2 + H2O → HOCl + HNO3
(22) HOCl + HCl → Cl2 + H2O
These reactions allow chlorine to be more easily photolyzed as Cl2 [13]. Additionally, nitrogen that could form ClONO2 species, which break the catalytic cycle of the chlorine radical, is used up by these reactions 20-22 [13].
Molina and Molina [11] put forth a set of reactions suggesting that chlorine from CFC’s was the main culprit in ozone depletion over Antarctica. Only this time, the reactions involve neutral molecules in the PSC’s denoted M in reactions [3,11,13, 21]:
(23) 2ClO + M → Cl2O2 + M
(24) Cl2O2 + hν → 2Cl + O2
(25) 2(Cl + O3 → ClO + O2)
(26) Net: 2O3 + hν → 3O2
This reaction involves the new species chlorine peroxide. The reactions were first verified to be the mechanism of ozone depletion over the poles by Solomon et al. in 1986. Further evidence came with a series of experiments from 1986-1987 and CFC release eventually was proven responsible for two thirds of the polar ozone depletion13. It is amazing that CFC’s released from industrial nations in the northern hemisphere can destroy 99% of the ozone over remote Antarctica thousands of miles away! Luckily, steps are being taken to control the release of CFC’s.
CFC Regulation
Greenhouse Gas verses Ozone Depleting Gas:
It should first be noted that a greenhouse gas is not the same thing as an
ozone depleting gas. An ozone depleting
gas, like CFC-12, destroys ozone. A
greenhouse gas, like CO2, holds warm air close to the earth’s
surface. There is strong, verified
evidence that ozone destruction takes place (see above), but it is difficult to
tell whether global warming is actually taking place.
The reason that it’s so difficult to verify global warming is because we know it’s already happening16! Take the moon for example: it is a known fact that the moon has no greenhouse effect and because there is no gas to retain heat it is very cold on the moon. The global warming effect on earth, due to “greenhouse” gases in the atmosphere, can be linked to about a 33°C rise in temperature16. So, normal global warming is good because we’d all die without it! The question is whether the gases we are putting into the atmosphere are enhancing this warming effect to the point where our climate and lifestyle will be affected.
Laws: Substances in the atmosphere are assigned two numbers: a global warming potential (GWP) with CO2 set arbitrarily to one and an ozone depletion potential (ODP) with CFC-11 set arbitrarily to one [21]. These can be expressed mathematically as follows [21]:
ODP = ΔO3(x)/ΔO3(CFC-11)
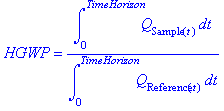
Dichlorodifluoromethane control began in 1979 with an EPA ban on Freons used for cosmetic propellants [21]. The discovery of the ozone hole in 1985 has further driven regulation [21].
The first major international law signed into effect to deal with the release of CFC’s and ozone depletion was the Montreal Protocol, signed in fall of 1987 [19] in Montreal Canada [21]. The treaty and its amendments phase out the use and production of ozone-depleting chemicals with a ten year grace period for developing countries. Industrial nations stopped using CFC’s in large amounts in 1996 and “developing” nations such as Mexico are supposed to stop use by 2010 [19, 21].
The second major international law is the Kyoto Protocol signed in 1997, which seeks to restrict emission of greenhouse gases over a period of time and restrict ODP’s missed by the Montreal Treaty. However, treaties can hurt a country’s bottom line; especially when there isn’t enough convincing evidence to back up the greenhouse effect theory. Thus, the United States has refused to sign up for Kyoto until the evidence is more concrete; we are a knee jerk reaction society after all.
Dichlorodifluoromethane Alternatives: Although
production is stopped, pre-ban CFC-12 can still be purchased new in the box at
approximately $500 for thirty lbs, but chemists are on the lookout for good
CFC-12 alternatives.
CO2 is recommended to replace CFC’s for blowing applications21, but all of the refrigeration factors discussed above must be accounted for when looking for new refrigerants.
Currently, chlorofluorocarbons are being phased out and replaced with fluorocarbons. The idea is that if there isn’t chlorine in the molecule, then it won’t be able to destroy any ozone (giving an ODP of zero). However, the drawback is that C-F bond absorbs far more infrared radiation than even CO2 [8], thus these new fluorocarbons generally have a high GWP. Take FC-116 (C2F6) for example, it is emitted to the atmosphere by the Hall aluminum process and sports an atmospheric life of 10,000 years with a GWP of 95008.
The ideal alternative refrigerant has all of the properties previously listed, is non-toxic, has a low vapor pressure at the right temperatures, doesn’t destroy any ozone, and doesn’t absorb very much infrared radiation. This is a hard bill to fit, but chemists are up to the challenge.
According to SUVA, Dupont’s refrigeration division, the suitable legal alternative to CFC-12 is R-134a1, 8, 21. Both ODP and GWP must be kept in mind when looking for alternative refrigerants, and ideally both ODP and GWP would be zero. R-134a has a formula CF3CH2F and a GWP100 Years of 1300 [6,21] and an ODP of zero (it doesn’t have any chlorine to release). CFC-12 has a GWP100 Years of about 8500 [8, 21], so R-134a is probably a good trade in terms of environmental impact.
Hydrocarbons, such as propane, butane, iso-butane, cyclohexane, and cyclopentane have also been used as environmentally safe refrigerant gases after refrigeration process redesign21.
Conclusions:
We need refrigeration and ice to maintain our society. However, we must be careful what we put into the atmosphere. Atmospheric chemistry and atmospheric modeling is highly complex, and though climate change is very slow, there may be more “ozone-hole” type surprises. Now is the time to think about doing something about the problem and the Montreal Treaty and Kyoto Protocol are definitely headed in the right direction. Keeping earth habitable is really up to everyone. As far as dichlorodifluoromethane goes, we’re lucky that we have excellent chemists and scientists on the problem.
References:
(1) About SUVA Refrigerants: History Timeline 1910: The "Ice Age". http://www.dupont.com/suva/na/usa/about/history_1910.html (March 6, 2005).
(2) Althouse, John V. Auto Air Conditioning Technology, Goodheart-Willcox Company, Inc.: South Holland, IL 1991; pp 32, 45, 47, 57.
(3) Crutzen, Paul J. My Life with O3, NOx and Other YZOxs.
Nobel Lectures, Chemistry 1991-1995, Editor Bo G. Malmström,
World Scientific Publishing Co.: Singapore, 1997;
pp 189-241.
(4) Dominguez, Olga M. NASA Comment and Request for Exemption from EPA Proposed Rule. Protection of stratospheric ozone; Listing of Substitutes for Ozone- Depleting Substances. http://www.nasa.gov/pdf/45326main_hcfc1_001.pdf (March 6, 2005).
(5) Daudt H. W.; Kinetic Chemicals Inc. Organic Fluorine Compounds. 2,005,706, June 18, 1935.
(6) EPA Global Warming: Annex S: Global Warming Potential Values. http://yosemite.epa.gov/oar/globalwarming.nsf/UniqueKeyLookup/LHOD5MJTD8/$File/2003-final-inventory_annex_s.pdf (March 6, 2005).
(7) Geiger, Franz M.; Charles, Pibel D.; Hicks, Janice M. The Hydrolysis of Chlorine Nitrate on Ice Is Autocatalytic. J. Phys. Chem. A 2001, 105, 4940-4945.
(8) Gumprecht, William. Kennesaw State University: General Chemistry Case Studies: (Refrigerants for the 21st Century). http://chemcases.com/fluoro/index.htm (March 6, 2005).
(9) Krisch, M. J.; et al. Photodissociation of CH3OCl to CH3O + Cl at 248 nm. J. Phys. Chem. A 2005, 108, 1650-1656.
(10) Lamere, Bernard. How Cooling Equipment Works. Guide to Home Air Conditioning and Refrigeration Equipment, John F. Rider Publisher, Inc.: New York, 1963; pp1-13.
(11) Molina, L.T.; M.J. Molina. Production of Cl2O2 from the self-reaction of the ClO radical, J. Phys. Chem. 1987, 91, 433,.
(12) Molina, M.J.; Rowland F.S., Stratospheric Sink for Chlorofluoromethanes:
Chlorine Catalysed Destruction of Ozone
, Nature 1974, 249, 810-814.
(13) Molina, Mario J. Polar Ozone Depletion. Nobel Lectures, Chemistry 1991-1995, Editor Bo G. Malmström, World Scientific Publishing Co.: Singapore, 1997; pp 250-263.
(14) National Safety Council: Chlorofluorocarbons Chemical Backgrounder. http://www.nsc.org/library/chemical/chlorofl.htm (March 6, 2005).
(15) Newman, Paul A.; et al. NASA: The Stratospheric Ozone Electronic Textbook.
http://www.ccpo.odu.edu/SEES/ozone/oz_class.htm (March 6, 2005).
(16) Palfreman, Jon. NOVA/Frontline (What’s up with the weather?): Interview with Richard C. J. Somerville. http://www.pbs.org/wgbh/warming/debate/somerville.html (March 6, 2005).
(17) Rowland, Sherwood F. Nobel Lecture in Chemistry. Nobel Lectures, Chemistry 1991-1995, Editor Bo G. Malmström, World Scientific Publishing Co.: Singapore, 1997; pp 273-296.
(18) Scott Specialty Gases. Material Safety Data Sheets (Dichlorodifluoromethane). http://www.scottecatalog.com/msds.nsf/0/72d85a355ffe0cb785256a0a004e32fc?OpenDocument (March 6, 2005).
(19)
Smith, Michael; Vincent, Mary. Tanking a Killer Coolant. Canadian Geographic Sep/Oct 1997, Vol. 117, Issue 5, p 40.
(20) Young, Hugh D.; Freedman, Roger A. The Second Law of Thermodynamics. University Physics with Modern Physics, Pearson Education, Inc. & Addison Wesley: San Francisco CA 2004; pp 761-763.
(21) Wachowski, L.; et. al. Ecological Replacements of Ozone-Depleting Substances. Polish Journal of Environmental Studies 2001 vol. 10, issue 6, p 417.
(22) Yves A. Mantz; et al. A Theoretical Study of the Interaction of HCl with Crystalline NAT. J. Phys. Chem. A 2002, 106, 6972-6981.