Oxidation Reduction Chemistry of the Elements
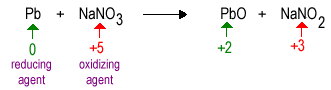   |
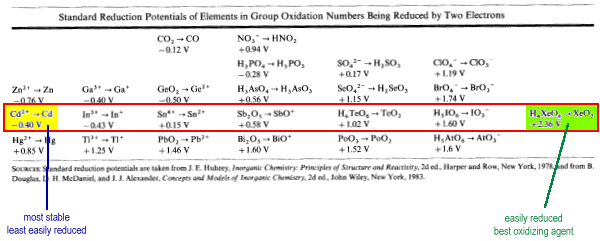
Periodic Trends in Standard Reduction Potentials of Oxo Anions and Acids
| The less positive the value of the standard reduction potential (Eo), the more stable the species is and the less easily it is reduced. |
p-Block Elements
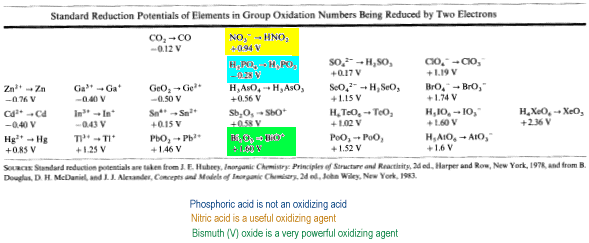
Consider oxo anions and acids of the p-block in their highest oxidation state (charge equal to group number) being reduced by two electrons.
As oxidation number increases across a Period from left to right, Eo becomes more positive. Therefore, stability decreases across the Period and the ability to act as an oxidizing agent increases.
d-Block Elements
The trends across a Period are the same as in the p-Block Elements while the trends down a family are different. In the d-block, the elements in Period 4 show the greatest reluctance to adopt the group oxidation number (the most easily reduced.)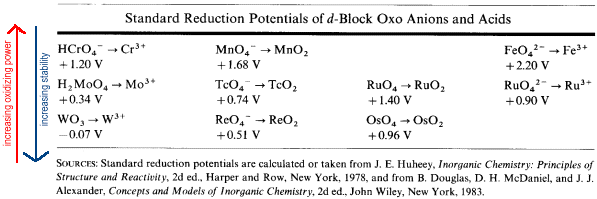
Redox Reactions and the Real World
Most redox reactions occur under nonstandard conditions. As a reaction proceeds, the concentrations of reactants and products change altering the driving force for the reaction.The effect of nonstandard concentrations on potential at room temperature is given by the Nernst equation.
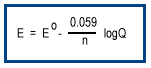
Consider the reduction of a metal ion to a metal.
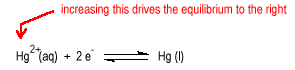
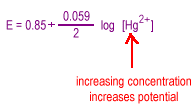 |
| Increasing the concentration of the metal ion will tend to drive the reaction to the right increasing the potential |
The Synthesis of Oxo Anions
The dependence of potential on concentration is more complicated for oxo anions, oxo acids, oxides and hydroxides than it is for simple metal cations. Consider the reduction of ferrate to ferric ion.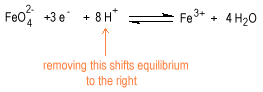
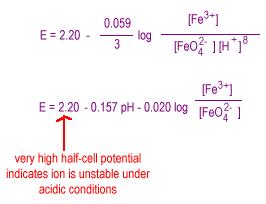
Decreasing the concentration of the hydrogen ion shifts the equilibrium to the left favoring ferrate stability in strongly basic solution.
Practical Consequence:
The synthesis of a salt of an oxo anion with a very highly oxidized central atom is normally carried out in basic solution.
| Oxo anions salts with very highly oxidized central atoms tend to be useful oxidizing agents which work most effectively in acidic solutions. |
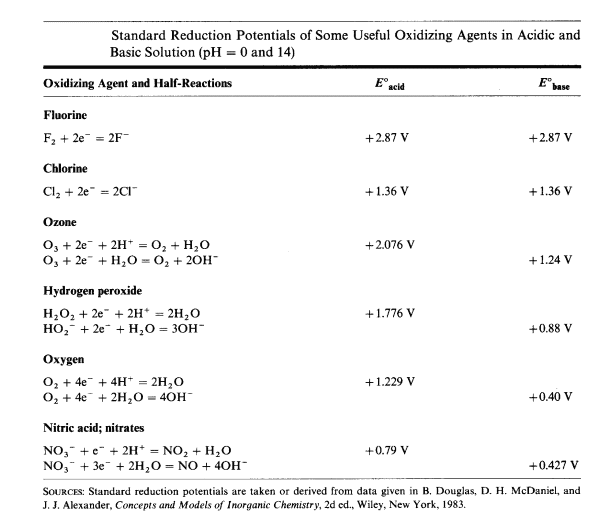
To carry out oxidations in basic solution, an oxidizing agent such as chlorine that involves few or no hydrogen ions in the reduction is preferred.
| Typically, for species with standard reduction potentials greater than +1.2 V in acidic solution, the use of strongly basic solutions and strong oxidizing agents is required. |
A very unstable species such as the ferrate ion is normally prepared in strongly alkaline solution, using chlorine as the oxidizing agent.
The equilibrium is driven to the product side by precipitating the ferrate ion with an appropriate cation (Ba2+ is a good choice since the ferrate ion is feebly basic).
Hydrogen peroxide is another oxidizing agent that can be used in alkaline solution.

| Oxo Anion | Starting Material | Oxidizing Agent |
| periodate | iodate | chlorine |
| tellurate | tellurite | chlorine |
| perxenate | xenon trioxide | ozone |
| perbromate | bromate | fluorine |
Many oxo anions can be prepared by the electrolysis of aqueous solution containing the elements in low oxidation states. In these cases, water is reduced at the cathode generating hydroxide ion lowering the pH while the oxo anion is produced at the anode.
| Oxo Anion | Starting Material |
| hypochlorite | aqueous sodium chloride |
| permanganate | manganate ion |
If the oxo anion has a standard reduction potential in the range +0.3 to +1.2 V, basic conditions are not usually necessary and strongly oxidizing acids such as nitric acid may be used as the oxidizing acid.
| Oxo Anion | Starting Material |
| iodic acid | iodine |
| selenous acid | selenium |
| arsenic acid | As2O3 |
The element may also be oxidized in air generating its acidic oxide. This acidic oxide may be converted into its oxo acid by reaction with water or directly to the oxo salt by reaction of the acidic oxide with a basic oxide.
If the desired oxo acid or anion has a reduction potential below about +0.2V, acid-base reactions may be sufficient since the element may well occur naturally in the desired oxidation state.
| Syntheses for oxo acids and anions can be found in: Brauer, G. ed. Handbook of Preparative Inorganic Chemistry, 2 vols, 2nd ed., Academic Press, New York, 1963. |