Hydrolysis of Oxoanions
Oxo Anion Hydrolysis
Like cations, oxoanions are hydrated in aqueous solution. In this case the electrostatic attraction is between the electron pairs on the oxoanion oxygen atoms and the partially positive hydrogen atoms of the water molecule. The hydration of oxoanions is an exothermic process. The hydration energy is dependent on the charge and size of the oxoanion. Hydration energies increase with increasing charge and decreasing anion size.As with cations, if the interaction between the anion and the hydrogen of the water is sufficiently strong, the hydrogen can be removed from the water generating a hydroxide ion resulting in a basic solution.
The equilibrium constant for this reaction is the base ionization constant, Kb.
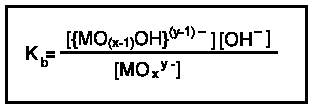
Base ionization constants are tabulated as pKbs. Successive ionizations are listed as pKb1, pKb2, etc. The larger the pKb value the lesser the degree of ionization and the weaker base the oxoanion is.
When determining the acidities of metal cations, three variable are important -- charge, size and electronegativity of the cation. When determining the basicity of an oxo anion, the size of the nonmetal atom (or high oxidation state metal atom) is not relevant. This central metal atom is significantly smaller than the multiple oxygen atoms in the anion. Therefore, different central atoms bearing the same number of oxygens will be very close in size.
Effect of Charge on Basicity
Increasing charge on an anion increases its tendency to hydrolyze and form basic solutions.| Anion | pKb1 | pKb2 | pKb3 |
|---|---|---|---|
| H3AsO4 | 10.5 | 6.8 | 1.5 |
| H3PO4 | 11.88 | 6.8 | 2 |
| H2SeO3 | 11.43 | 7.4 | |
| H4GeO4 | 5.41 | 1 |
The table above shows that the pKb values of an oxoanion decrease by 4-5 units for each additional negative charge on the anion. Thus, increasing the negative charge substantially increases the anion's basicity.
Effect of Number of Oxygen Groups
Since most nonmetals exhibit more than one oxidation state, they can form oxoanions that differ in the number of oxygens bonded to the metal. The different oxoanions of a given nonmetal atom will differ substantially in their basicities. For example, chlorine forms four different oxoanions: ClO-, ClO2-, ClO3-, ClO4-. ClO- has a pKb of 6.5; ClO2- 12.1 while the pKb value of the other two oxoanions are such weak bases that their hydrolysis in not measureable. Adding additional oxygens decreases the basicity of the oxoanion. Each additional oxo group increases the pKb value by about 5.7 units.Effect of Electronegativity
As the electronegativity of the nonmetal atom decreases the basicity of the oxo anion increases. Comparing the pKb values orClO-, 6.50; BrO-, 5.3; IO-, 3.4 demonstrates this trend.Reasonably accurate pKb1 values can be obtained via the following equation where x is the number of oxo groups and y is the number of units of negative charge.
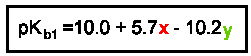
|
The table below lists calculated values of pKb1 for the important simple oxo anions of the elements.
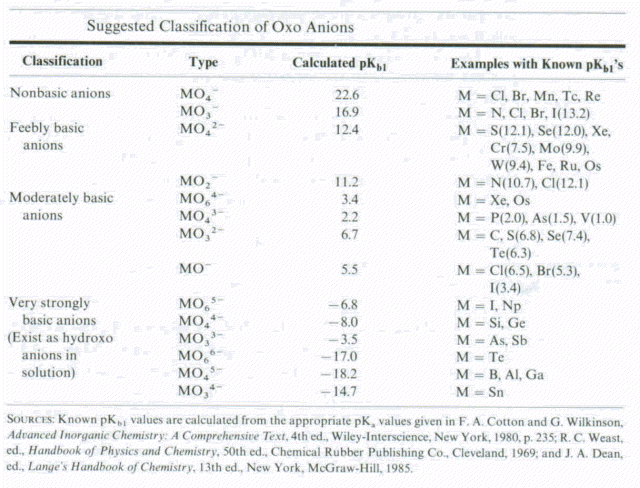 Table copied from Wulfsberg,G. Principles of Descriptive Chemistry; Brooks/Cole Publishing:Monterey CA, 1987; p.37.
Table copied from Wulfsberg,G. Principles of Descriptive Chemistry; Brooks/Cole Publishing:Monterey CA, 1987; p.37.
Oxo anions can be placed into categories that describe the extent to which they hydrolyze. These categories are analogous to those used with cationic species. Nonbasic anions do not hydrolyze appreciably. Feebly basic anions produce small amounts of hydroxide ion which are not easily detected in the laboratory. The anions in these categories are typically salts of strong and fairly strong acids. Moderately basic anions give distinctly basic solutions which can be detected using test papers. The salts of weak acids which you studied in general chemistry fit into this category. Very strongly basic anions hydrolyze nearly completely in water.
Predicting Basicities
A rough extimate of the basicity of an oxo anion can be made by inspecting its chemical formula.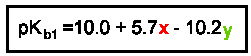
Note from the equation that adding an additional negative charge has twice the effect on the pKb as addition of an oxo group. In other words, the addition of a negative decreasesthe pKb value twice as much as adding an oxo group will raise it. This observation can be used to categorize the oxo anion's basicity. To do this you will remove the charges and oxo groups that counteract each other and use the resulting "formula" to determine the base category of the oxo anion. In general, anions containing a excess of oxogroups will not be very basic; anions with an excess of negative charge will be strongly basic.
| Classification | Resulting Formula |
|---|---|
| Nonbasic | contains oxo groups & no charge |
| Feebly basic | no charge & no oxo groups |
| Moderately basic | no oxo groups & a charge of -1/2 or -1 |
| Very strongly basic | no oxo groups & a charge more negative an -1 |
Examples:
Consider an oxo anion of formula MO4-. For this formula the effect of the unit of negative charge will cancel out the effect of two of the oxo groups. If you remove the negative charge and two oxo groups the resulting formula is "MO2". This anion falls into the category of nonbasic. According to the table of pKb values above, oxo anions of formula MO4- have high pKb values and do fall into the nonbasic category.
Consider an oxo anion of formula MO34-. The three oxo groups cancel 1.5 unit of negative charge. The resulting formula becomes "M-2.5". This oxo anion will be very strongly basic.
Classify the basicity of the following oxo anions and describe their reactions with water.
 Use the CRC to determine if the categories you have predicted are accurate. (HINT:
pKb values are rarely tabulated. You will have to look up the dissociation
constants for the conjugate acids of these species and determine pKb from there.)
Use the CRC to determine if the categories you have predicted are accurate. (HINT:
pKb values are rarely tabulated. You will have to look up the dissociation
constants for the conjugate acids of these species and determine pKb from there.)
|
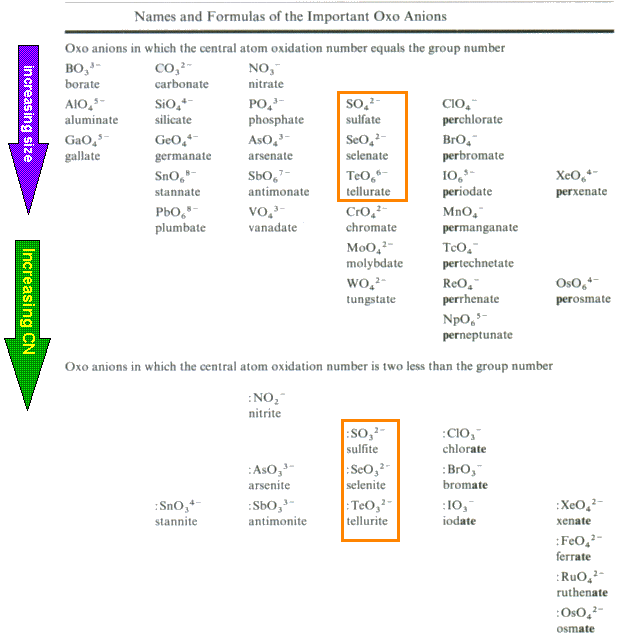
Formulas of Oxo Anions
A wide variety of oxo anions exist. The number of oxo groups present in an oxo anion is dependent on the nature of the central atom M.
Size of Central Atom
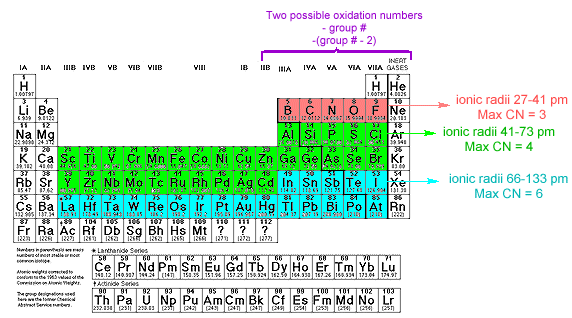
The p-Block Elements:There are two common oxidation numbers for elements in the p-block of the periodic table
- the group number
- the group number minus two
| Oxoanions in which the central atom has an oxidation number 2 less than the group number will possess at least one less oxo group than those in which the central atom has an oxidation number equal to the group number. |
You can see this trend by comparing the oxoanions in the two orange boxes on the Table above.
The d-Block Elements:
Oxidation numbers are not as predictible among the d-block elements. For these elements nonbonding valence electrons do not take up the space that would otherwise be occupied by an oxo group. (why?)
The term total coordination number is used to indicate the total number of atoms and unshared p-electron pairs around a central atom.
| In general, the number of oxo groups is not dependent on the oxidation number |
Summary of Trends in Numbers of Oxo Groups in Oxo Anions
- The oxo anions having the smallest central atoms are those of the Period 2 p-block. These can have a maximum coordination number of 3 and will accomodate either three oxo groups or two oxo groups and one unshared pair of p electrons.
- Central atoms of the p-block of Periods 3 & 4 and those of the d-block of Periods 4 & 5 have larger radii and can have a maximum coordination number of 4. If the valence orbitals are 3p, ed, 4p or 4 d, the central atom can accommodate four oxo groups and unshared p-orbital electrons.
- Central atoms of the p-block of Periods 5 and the p- and d-block of Period 6, have a maximum total coordination number of 6. If the valence orbitals on the central atom are 5p, 5d, 5f, 6p, or 6d, the central can accomodate 4-6 oxo groups and unshared p-orbital pairs.
The other important feature in the formula of an oxo anion is its charge. To calculate the charge, add the group number for the central atom plus the number of oxo anions at -2 charge.
Predict the formulas of the oxo anions formed by the following elements, each with the specified oxidation number:

|