Hydration and Hydrolysis of Metal Cations
Hydration
When sodium chloride dissolves in water, the sodium and chloride ions and the polar water molecules are strongly attracted to one another by ion-dipole interactions. The solvent molecules (water in this case) surround the ions removing them from the crystal and forming the solution. As the dissolving process proceeds, the individual ions are removed from the solid surface becoming completely separate, hydrated species in the solution.Dissolution of NaCl in Water
In order to dissolve the sodium chloride in the water, three processes have to occur:
- the water molecules must be separated (H-bonds must be broken)
- the forces of attraction between the ions in the NaCl lattice must be broken
- solute-solvent(ion-dipole)interations must be formed
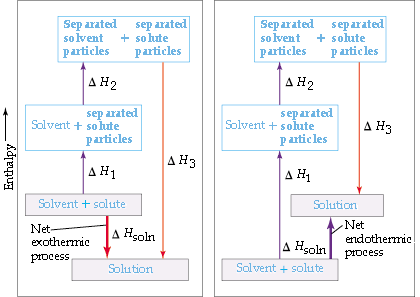
Whether the dissolution process will be exothermic or endothermic depends on the relative magnitudes of the energy changes for the three steps. In general, a substance will be insoluble if the energy expended to break apart the solvent and solute particles is significantly greater than the energy given off when solute-solvent interactions are established.
Hydration Energy
If you were to take gaseous cations and put them into water, they would form hydrated ions and release large amounts of energy. This energy is called the hydration energy of the cation. Although this is an experiment that is impossible to perform, the hydration energies have been determined indirectly (you will see how this is done later in the term).
|
As expected from Coulomb's Law
 Latimer's Equation
Latimer's Equation
In this equation Z is the charge on the cation and r is the cationic radius (in pm). The constant, 50, roughly equates to the radius of the oxygen atom in water.
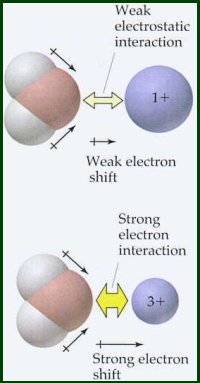
Latimer's equation does not include the effects of electronegativity. This equation is valid for cations that have electronegativities less than 1.5. Compare the hydration energy data for cations having electronegativities <1.5 and those having electronegativities >1.5. Cations with electronegativies >1.5 have substantially higher hydration energies than more electropositive cations of comparable radius and charge. These electronegative metals have some degree of covalent bonding in their interaction with the oxygen atom of the water molecule, i.e., oxygen's unshared pair of electrons is shared with the metal atom.
Hydrolysis
Metal ions in aqueous solution behave as Lewis acids. The positive charge on the metal ion draws electron density from the O-H bond in the water. This increases the bond's polarity making it easier to break. When the O-H bond breaks, an aqueous proton is released producing an acidic solution.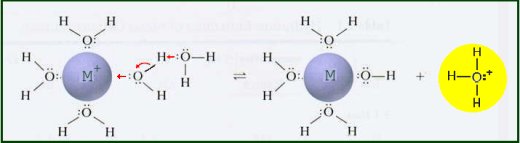 The equilibrium constant for this reaction can be measured.
The equilibrium constant for this reaction can be measured.
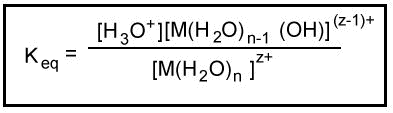
Note the similarity of the equation for the hydrolysis of a hydrated cation with the equation for the ionization of a weak acid in aqueous solution.
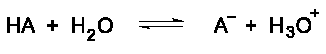
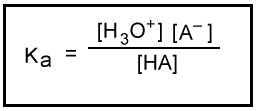
The Keq for the hydrolysis of a hydrated cation is analogous to the Ka for the ionization of a weak acid. Keq is an acid ionization constant. Generally, hydrolysis constants for cations are tabulated as -log Ka. These tabulated hydrolysis constants are averages of different experimental measurements that sometimes differ by more than one pKa unit. For this reason, small differences are not considered significant when comparing the values of these constants.

Use the data in the Table of Hydrolysis Constants for Metal Cations for these questions.
|
