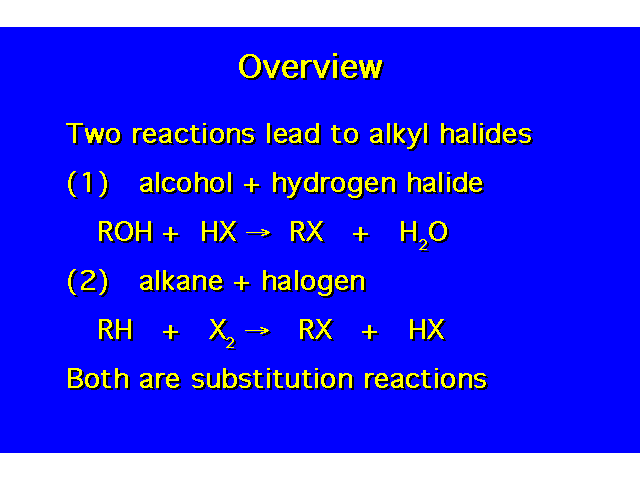
|
|
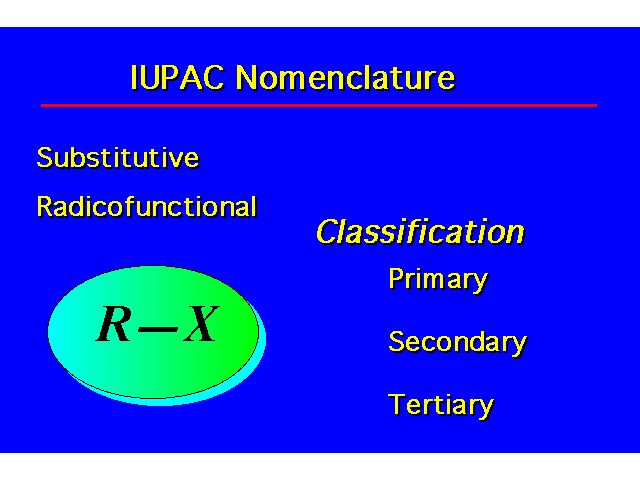
 | |
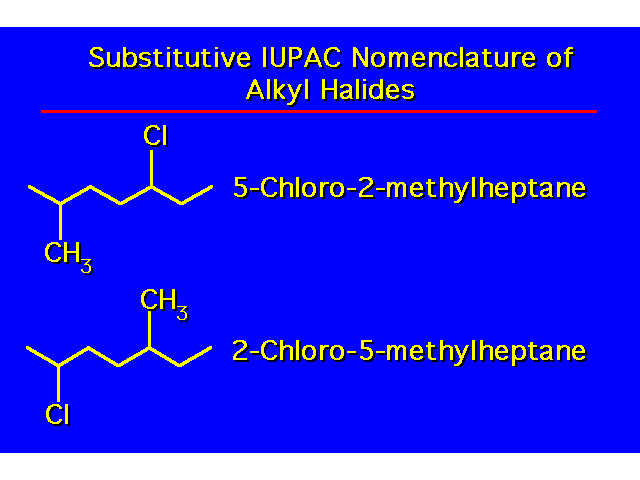
 | |
 | |
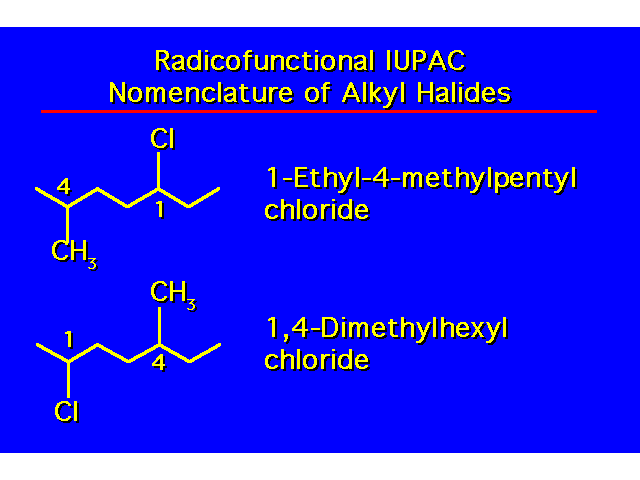
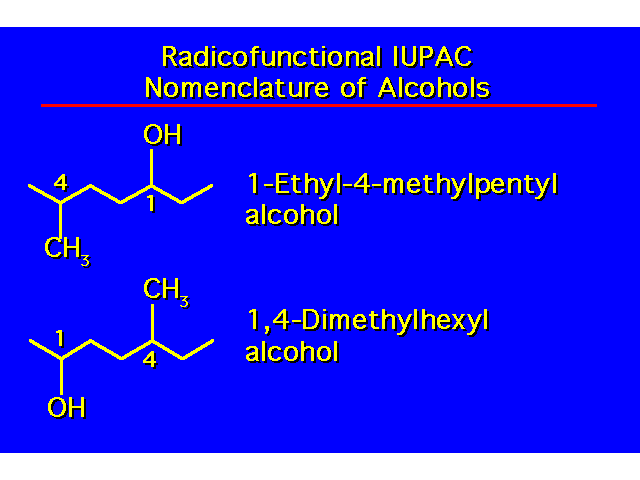
| -OH groups take precedence over halo and alkyl groups. Number the chain to give the -OH the lowest possible number. | 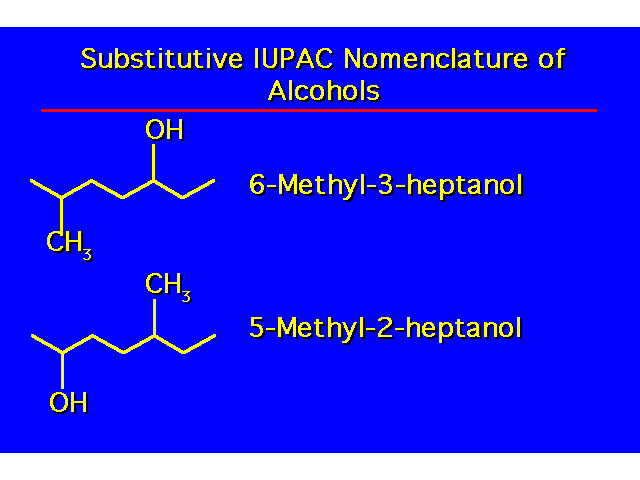 |
 | |
 | |
PHYSICAL PROPERTIESThe hydroxyl group is capable of donating and accepting a hydrogen bond. This causes alcohols to have properties that are very different to alkanes. | 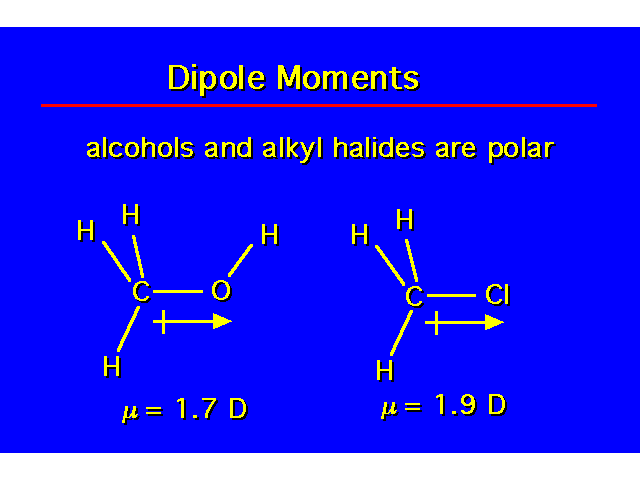 |
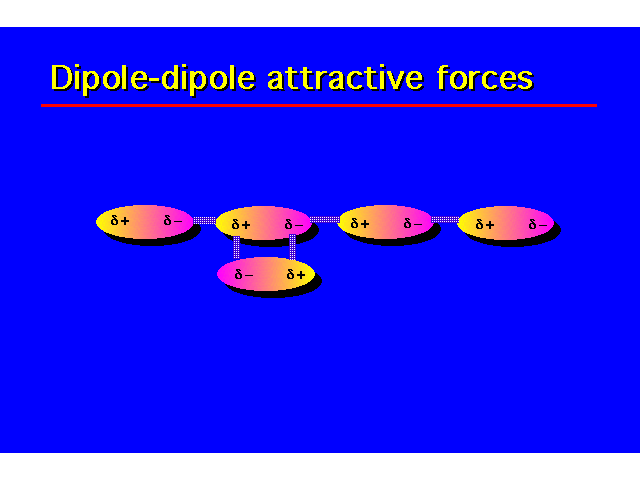 | |
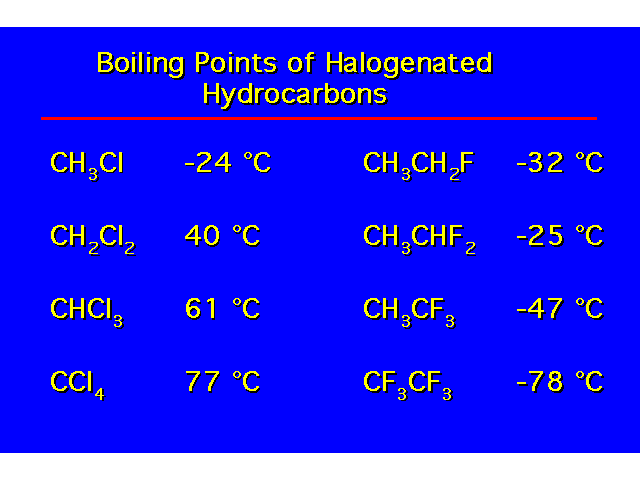
| H-bonding increases the coiling point and water solubility of alcohols over alkanes and alkyl halides. Alcohols containing 4 carbons or less are water soluble. Water solubility decreases with increasing length of the carbon chain. | 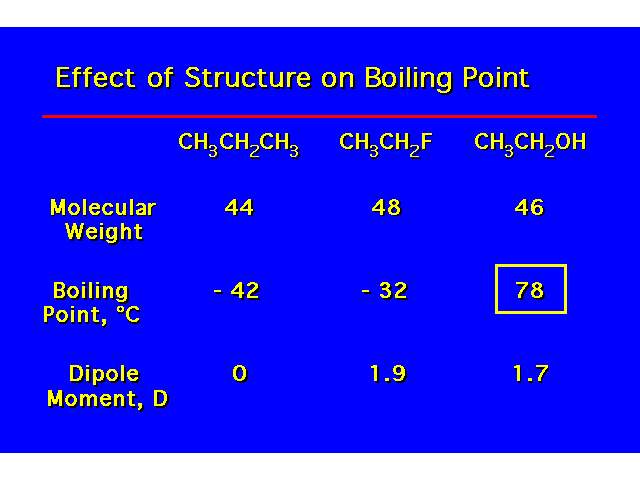 |
 | |
