|
|
Are the rings planar or nonplanar? | 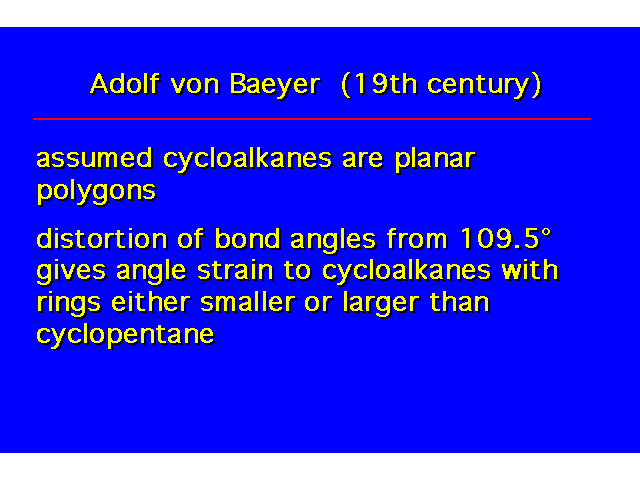 |
 | |
Cyclohexane has a heat of combustion that is about the same as for an unbranched alkane | 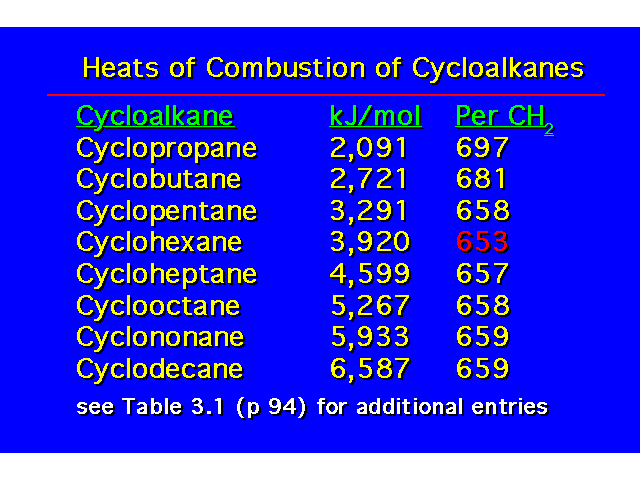 |
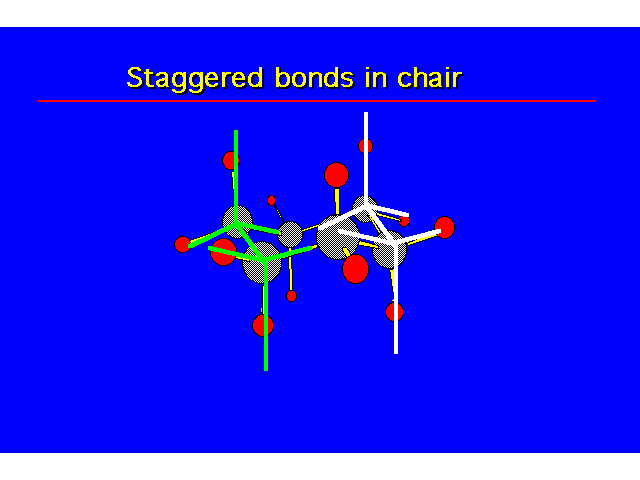
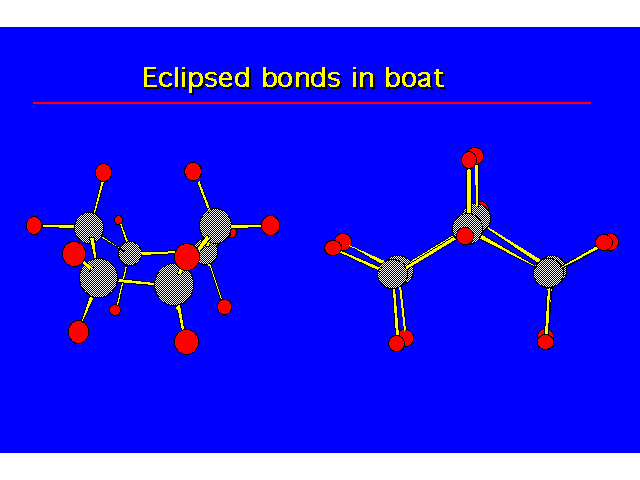
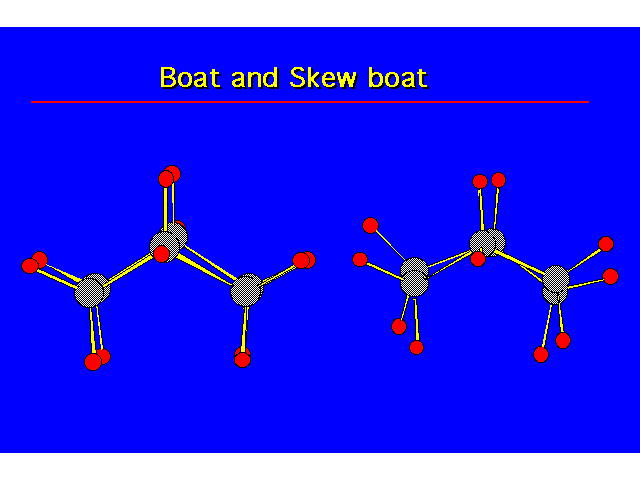
CONFORMATIONS OF CYCLOHEXANEHeat of Combustion Data suggests that cyclohexane is free of angle strain. To get tetrahedral geometries for the carbons, the ring must be nonplanar. | 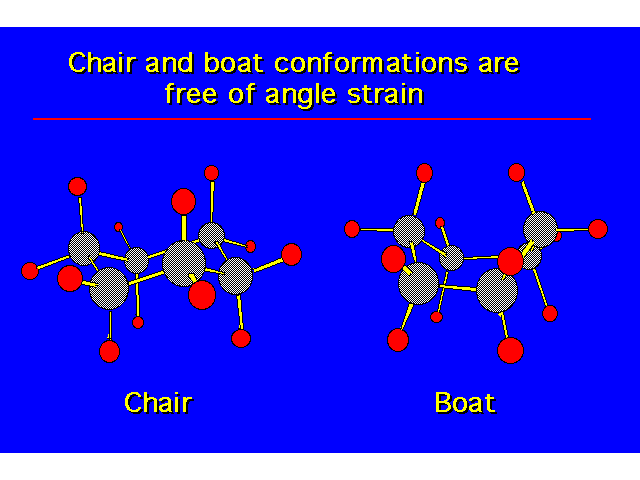 |
 | |
 | |
 | |
 | |
| 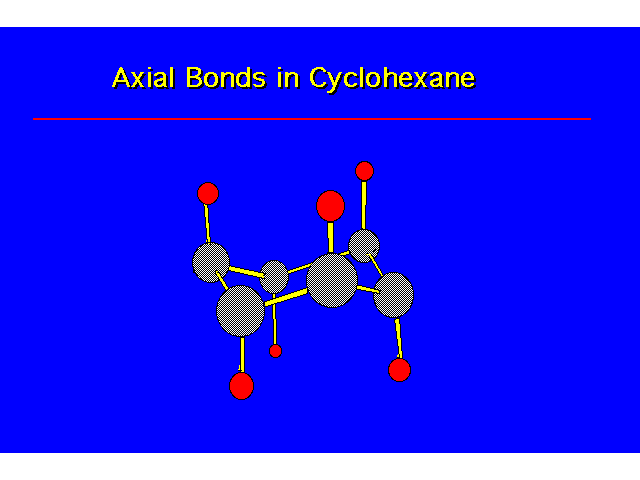 |
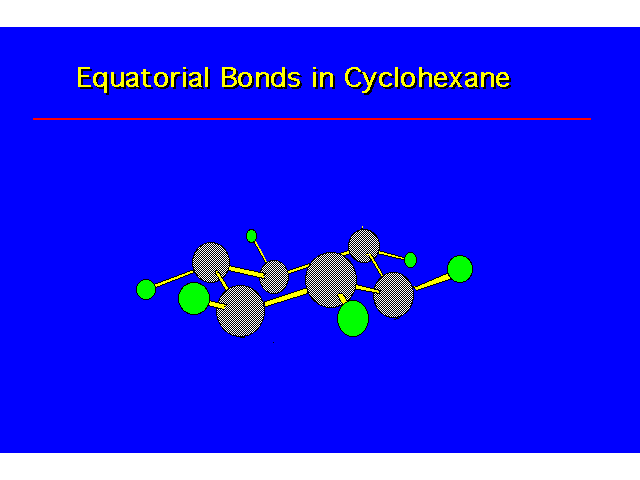 | |
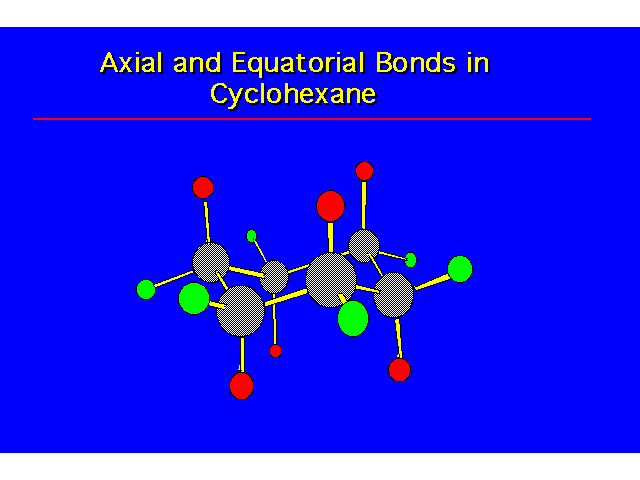 | |
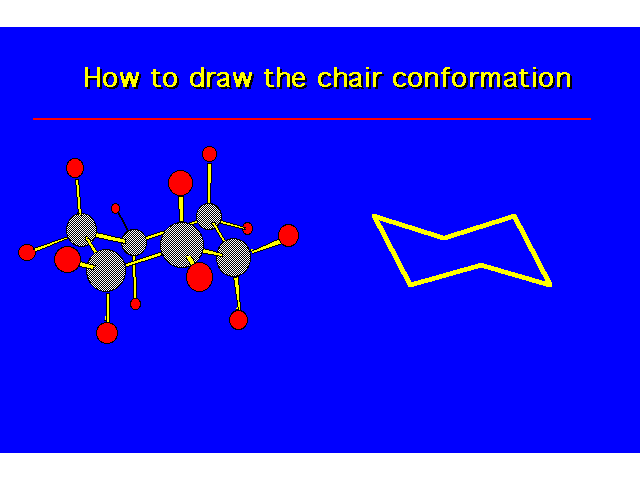 | |
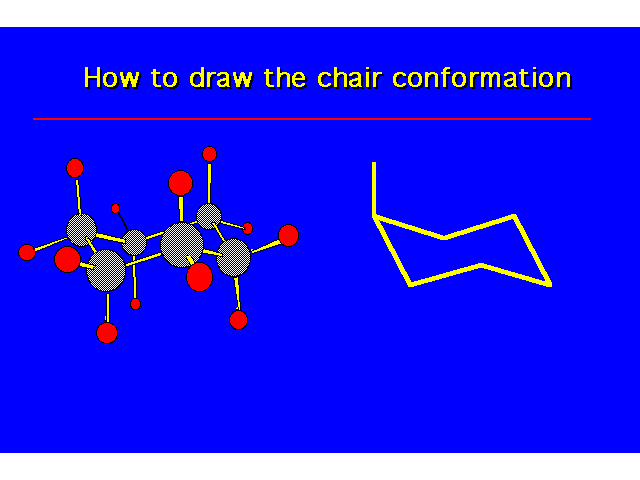 | |
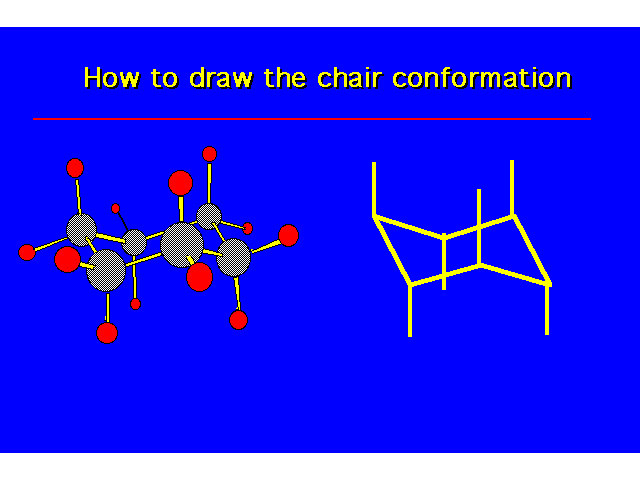 | |
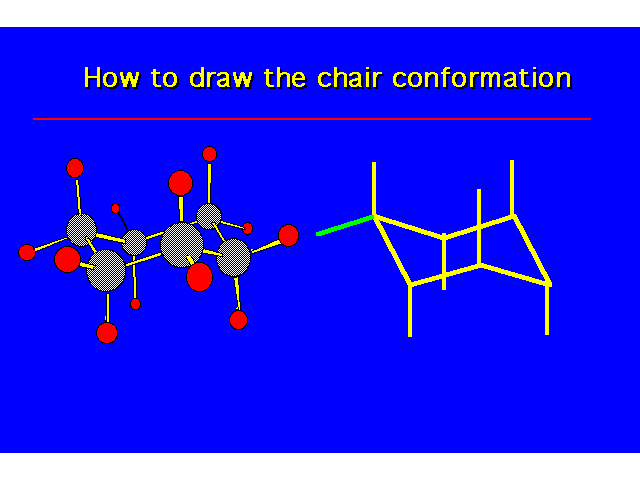 | |
 | |
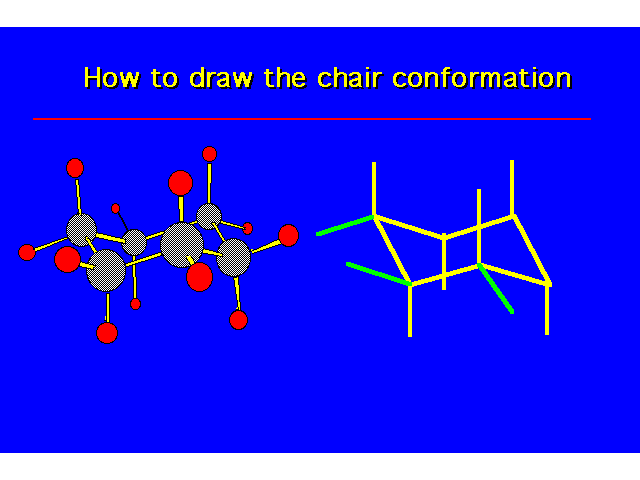 | |
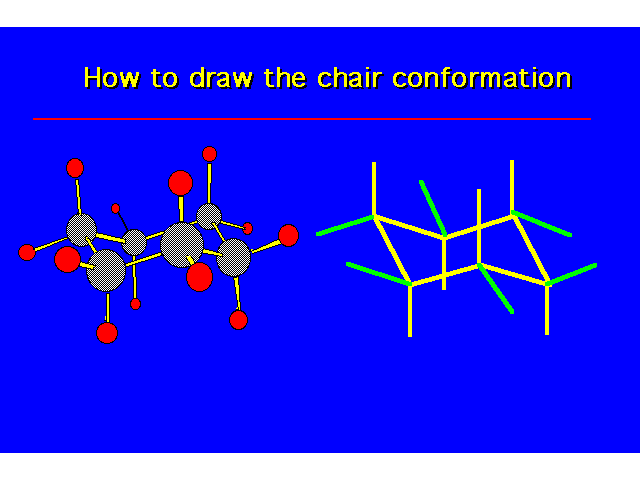 | |
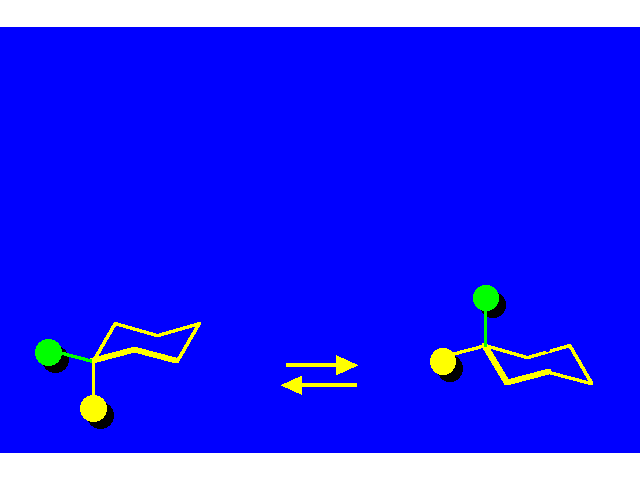
CONFORMATIONAL INVERSION OR "RING-FLIPPING" IN CYCLOHEXANE | 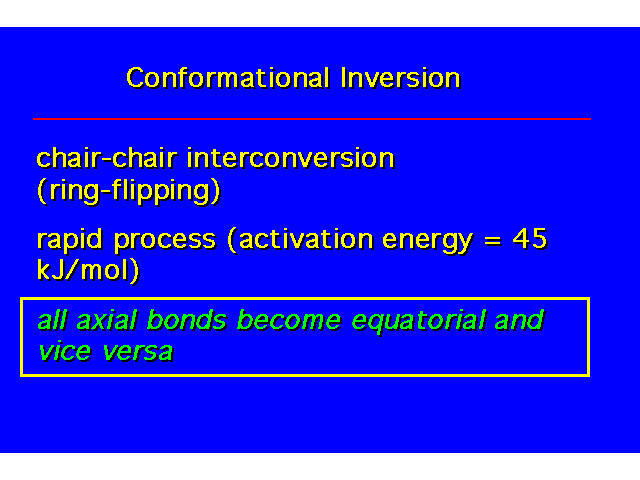 |
 | |
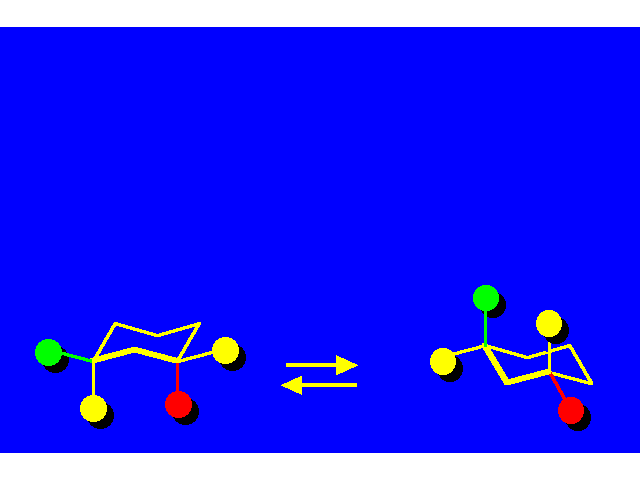 | |
In the ring-flipping process, the chair goes through the following conformations:
| 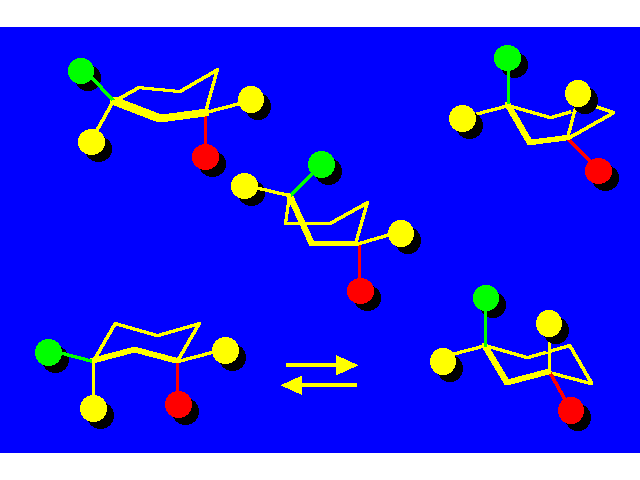 |
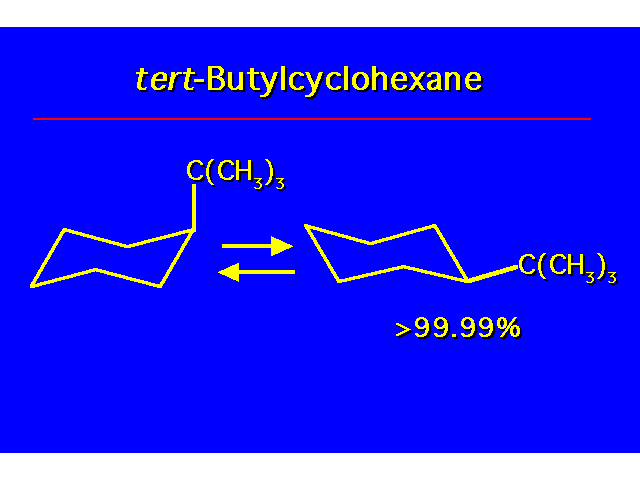
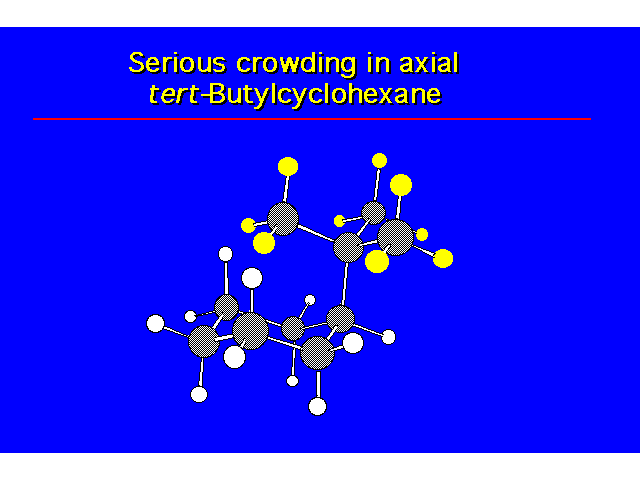
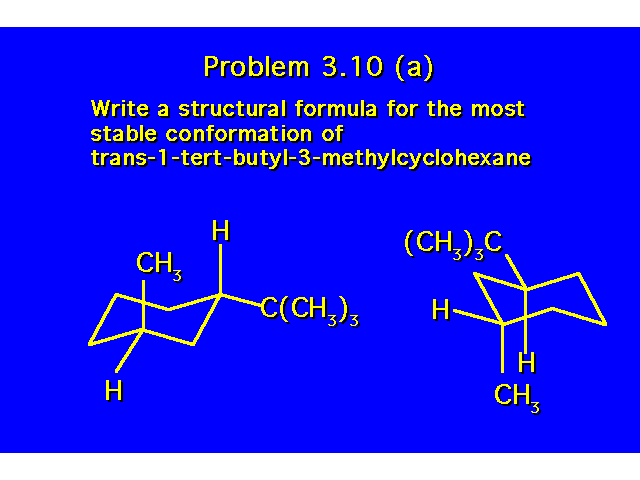
CONFORMATIONAL ANALYSIS OF MONOSUBSTITUTED CYCLOHEXANESThe most stable conformation is a chair with the substituent occupying an equitorial position | 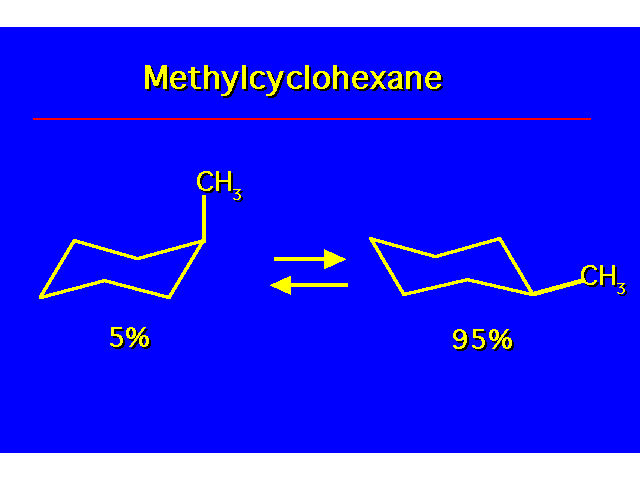 |
 | |
 | |
 | |
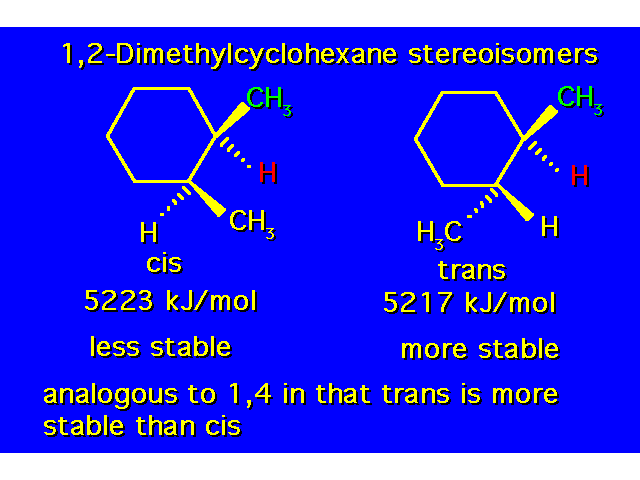
| Constitutional isomers differ in the order of their atomic connections. Stereoisomers have their atoms bonded in the same order, but they differ in the arrangement of atoms in space. | 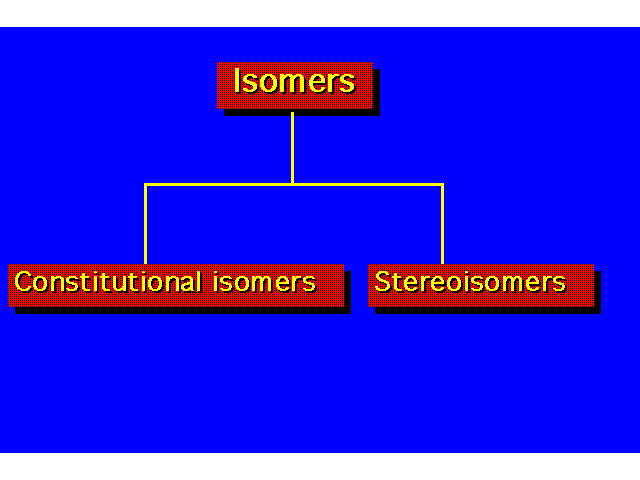 |
| The compound in the box exists as a pair of stereoisomers. | 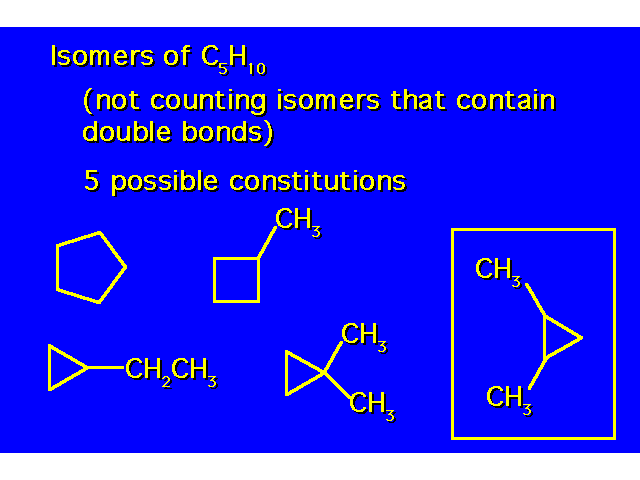 |
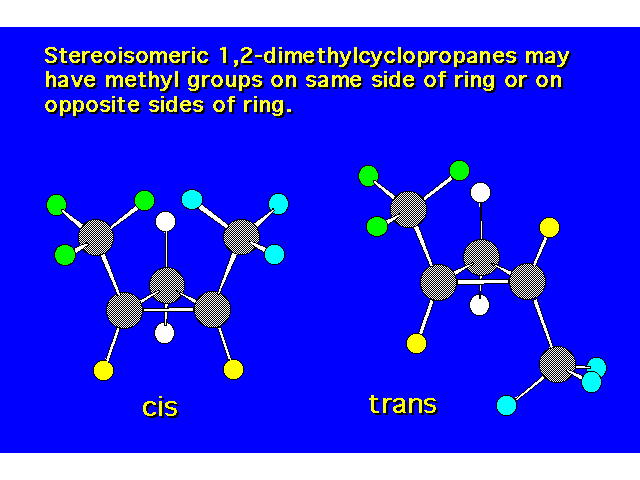 | |
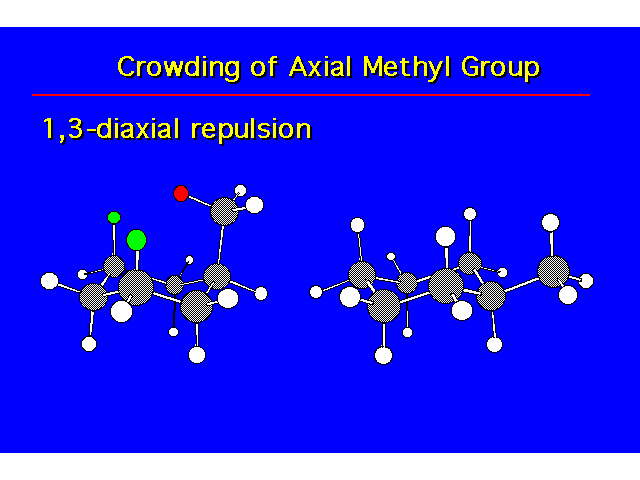
| The cis isomer is less stable due to steric crowding. | 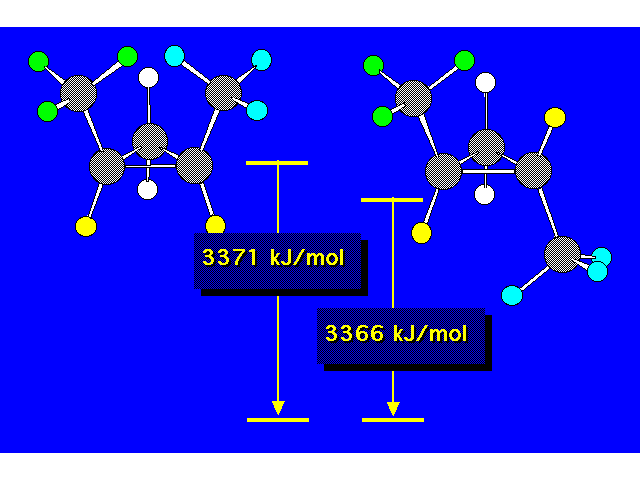 |
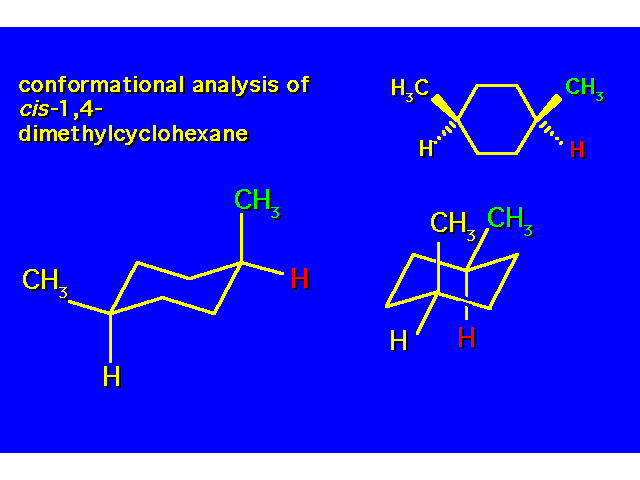
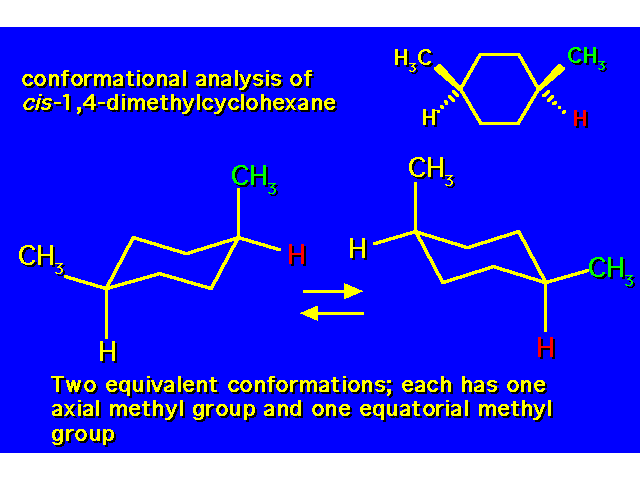
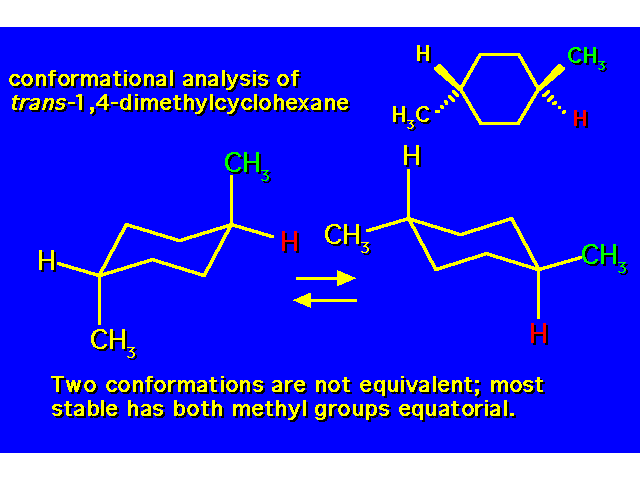
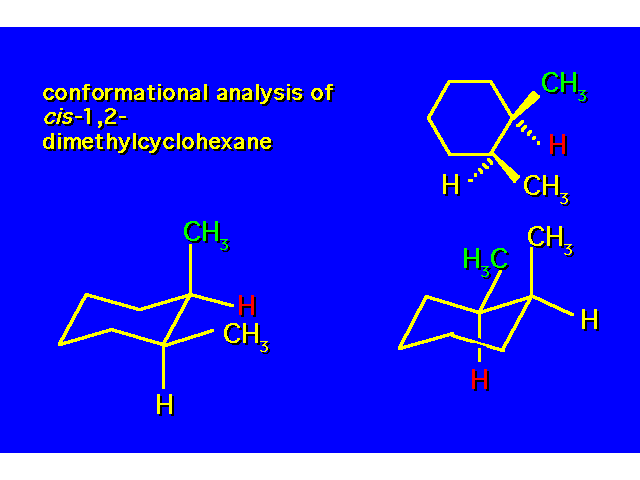
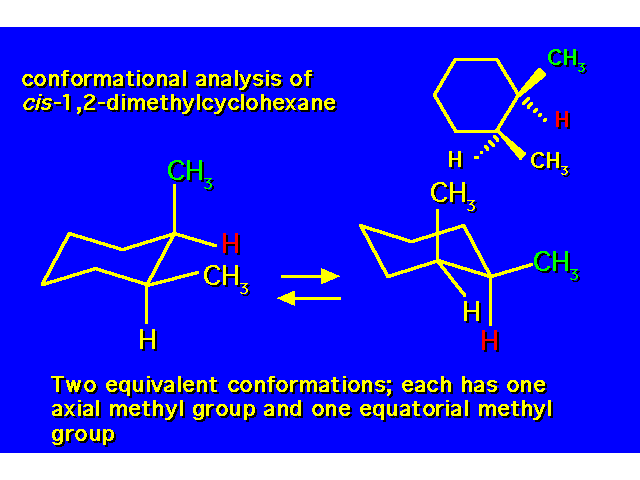
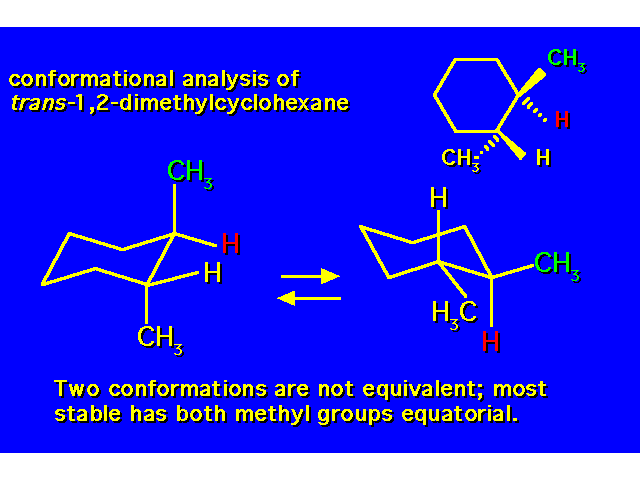
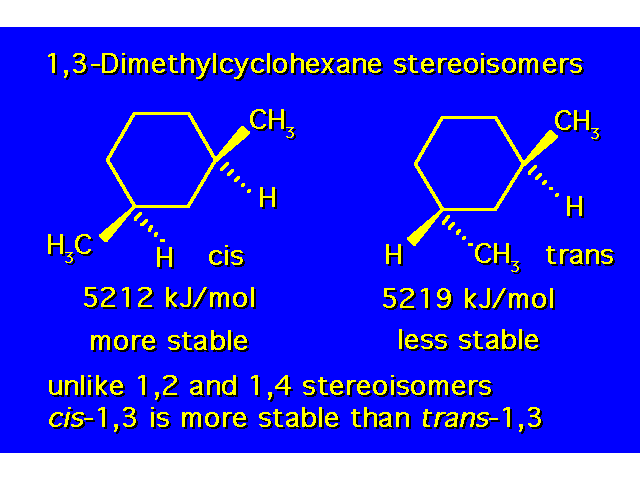
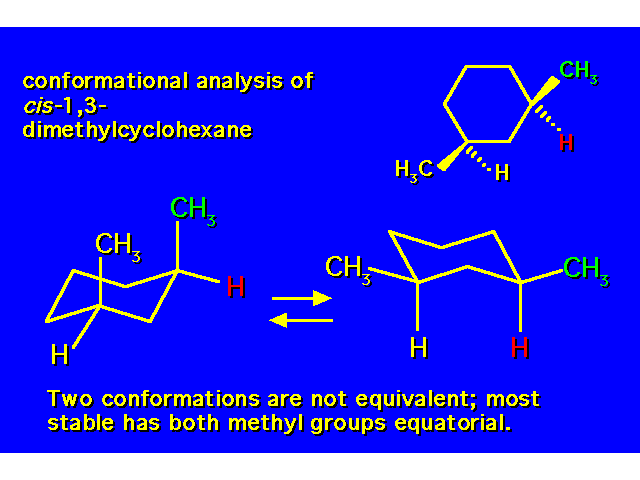
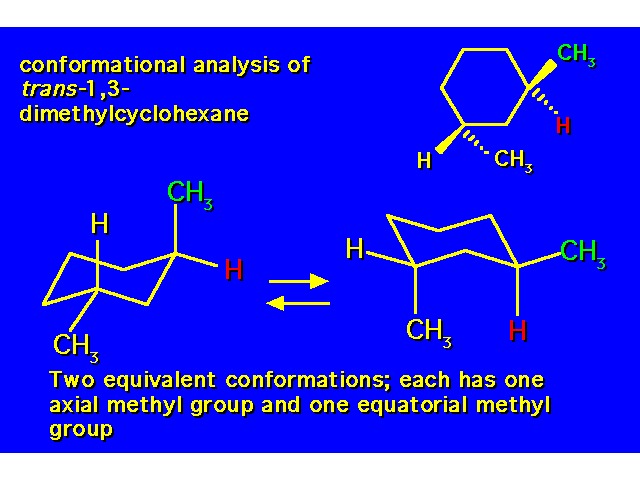
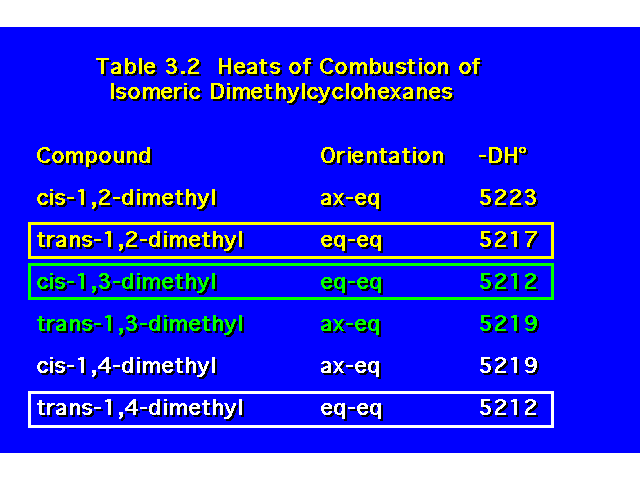
DISUBSTITUTED CYCLOHEXANES | 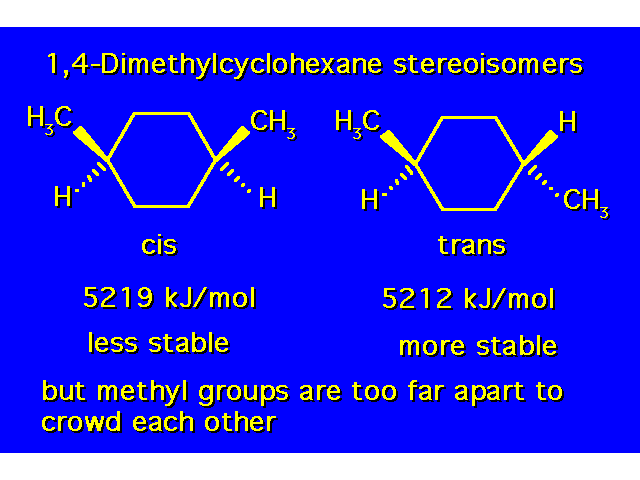 |
 | |
 | |
 | |
 | |
 | |
 | |
 | |
 | |
 | |
 | |
 | |
 | |
