Ch 334 Name _____________________________________
Midterm Exam #1
1.
(2 pts) What is the molecular formula of the
following molecule?
![]()
2.
(4 pts) Which of the following
describes the relationship between the two structures shown below. Circle the correct response.

identical
structures
resonance
forms
constitutional
isomers
different
compounds with different compositions
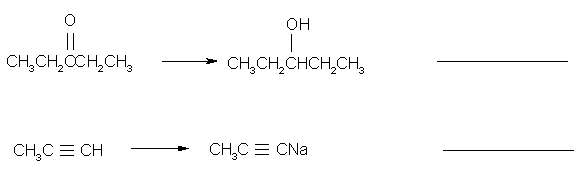
3.
(3 pts) Based on the VSEPR theory, which of the
following species has (have) a trigonal planar geometry? Circle them.
BCl3 NH3 NO3-
4.
(3 pts) Which of the following molecules would you
expect to have a dipole moment? Circle them.
CO2 HCN CHCl3
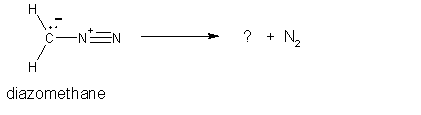
5.
(4 pts) Diazomethane is an unstable compound which
looses a nitrogen molecule when it decomposes.
What species is formed when the nitrogen molecule is lost? Circle your answer. (HINT:
draw the Lewis structure for the nitrogen molecule to help figure out
this question).
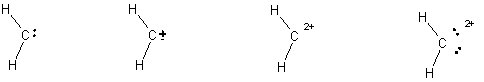
6.
(6 pts) (a)
What is the hybridization of the carbon atom indicated by the arrow in
the molecule limonene? Place your answer in the blank provided.
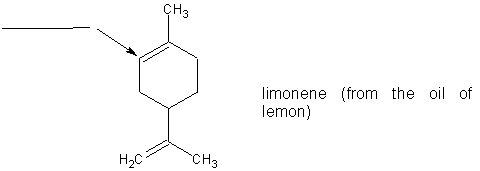
(b) There are ________ sigma bonds and ________ pi
bonds in limonene?
7.
(12 pts) Give the correct IUPAC names for the
following compounds.
CH3 CH3
![]()
![]()
![]() (a) CH3CHCH2CHCH2CH2CHCH3
(a) CH3CHCH2CHCH2CH2CHCH3
CH2CH3
![]()
(b) CH3CH2CH(CH3)C(CH3)3
![]()
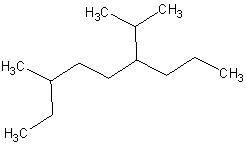
(c)
![]()
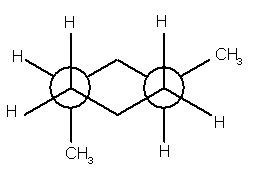
(d)
![]()
8.
(9 pts) Identify the relationship between the two
structures of each pair. Place the
letter of the appropriate response in the blanks provided.
A.
identical
B.
different
conformations of the same compound
C.
stereoisomers
D.
constitutional
isomers
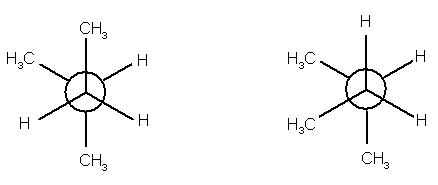
______ (a)
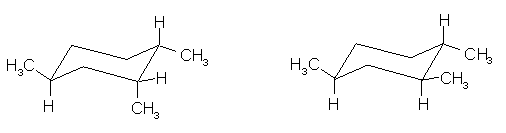
______ (b)
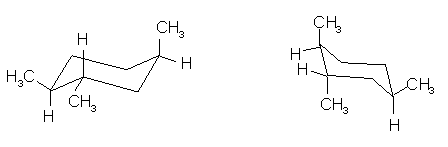
______(c)
9.
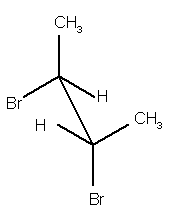 (6
pts) Use the sawhorse drawing of the
following molecule to answer this question.
(6
pts) Use the sawhorse drawing of the
following molecule to answer this question.
(a)
The two
bromine atoms in this structure have what relationship? Circle the correct response.
anti eclipsed gauche skewed
(b)
What is the
dihedral (torsion) angle between the two bromine atoms? Circle the correct response.
0o 30o 60o 90o
10.
(4 pts) Circle the statement that is correct
concerning the relative stabilities of the two conformations, A and B, shown
below.
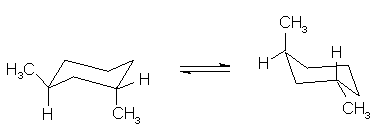
A
is more stable
B
is more stable
A
and B are equal in stabilities
A
and B are not equal in stability, but the preferred conformation cannot be
determined by inspection
11.
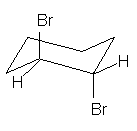 (4
pts) What is the spatial relationship of
the two bromine atoms in the following structure? Circle your answer.
(4
pts) What is the spatial relationship of
the two bromine atoms in the following structure? Circle your answer.
gauche anti eclipsed skewed
12.
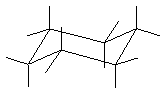
(4 pts) Draw the most stable conformation of trans-1-tert-butyl-3-methylcyclohexane. All you need to do is add the substituents
to the skeleton below.
13.
(4 pts) Which constitutional isomer of
dimethylcyclohexane does not exhibit cis, trans
isomerism? Circle your answer.
1,1-dimethylcyclohexane
1,2-dimethylcyclohexane
1,3-dimethylcyclohexane
1,4-dimethyl cyclohexane
14.
(4 pts) Circle the isomer of C5H10
that would be expected to have the highest heat of combustion.
methylcyclobutane cyclopentane
cis-1,2-dimethylcyclopropane trans-1,2-dimethylcyclopropane
15.
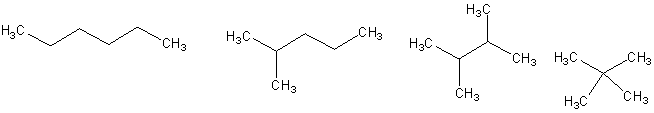
(4 pts) Circle the compound that would be expected to
have the lowest boiling point.
16.
(6 pts) Which is the stronger base: (Circle it)
(CHe)3N: (CHe)3P:
17.
(6 pts) Indicate whether the carbon-containing
compound in each of the following transformations undergoes oxidation,
reduction or has no change in oxidation state.