![[Western Oregon University]](/images/wouhead.gif)
Polysilicates
Compounds formed by the reaction of the acidic oxide silica (SiO2) with various basic metal oxides are of great importance in the geochemistry of the earth's crust.
These compounds contain silicon oxo anions which possess covalent Si-O bonds. Although the simple silicate ion SiO44- is sometimes found, many of these compounds have 2-coordinate oxygen atoms that link silicon atoms together into oligomeric or one-, two-, or three-dimensional polymers.
These complex silicates are the most common examples of polynuclear oxo anions.
Follow the links on this page to learn more about the chemistry and geology of silicates.

Background Information:
Some Mineral Basics
The Chemistry of Silicic Acid
Minerals by Chemical Composition
Silicates

Structures of Silicates
Silicate minerals are divided into subclasses according to their structures.
The basic structural types are:
The orthosilicates (single tetrahedrons)
Oligomeric Silicates
Cyclic Polysilicates
Chain Polysilicates
Sheet Polysilicates
Framework Polysilicates

The Orthosilicates
The orthosilicate ions SiO44- is a very strong base that cannot persist in aqueous solution. It tends to occur in nature as the insoluble salt of acidic cations. The orthosilicates are also referred to as the Nesosilicates.
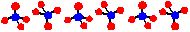
Oligomeric Silicates
The disilicate ion Si2O7 contains a 2-coordinate oxygen atom. When the oxygen from one unit becomes a bridge to the other unit, a terminal oxygen is removed. In this ion the charge density per silicon is lowered from the -4 in the orthosilicate to -3. The disilicate ion is an example of an dimer of the [SiO3]2- ion. The disilicate ion is less basic than the orthosilicate ion. Minerals containing the disilicate ion are fairly uncommon in nature and those containing larger oligomers are even more rare. These mineral are classified as sorosilicates.
Cyclic Polysilicates
Cyclic silicates form when the ends of chain structures join. The ring formation removes an additional terminal oxygen. In these structures each silicon has two bridging and two terminal oxygen atoms, and the charge density is -2 per silicon atom. Cyclic trimers [SiO3] 36- and hexamers [SiO3] 612-. These structures are sometimes called metasilicates and can be classified as cyclosilicates.
Chain Polysilicates
Single chain polysilicates form when a number of [SiO3]2- units link together into long chains. In these structures each silicon has 2 terminal oxygens and 2 bridging oxygens. The charge density is -2 per silicon atom. Single chain polysilicates comprise part of the subclass inosilicates.
Linear chains may be linked side-by-side into a double chain structures when a terminal oxide on silicon is replaced by terminal oxygen on another chain. When two chains are joined by bridging alternate SiO3 groups in each chain, a double chain structure [Si4O116n- results. Each silicon used to bind together the chains will have a charge density of -1 while those not linked will have a charge density of -2. These double chain structures are also classified as inosilicates.

Sheet Polysilicates
When side-to-side linking of chains is continued indefinitely, more oxides are eliminated yielding 2-dimensional polymers called sheet polysilicates of the formula [Si4O10]n4n-. A polysilicate sheet looks a bit like a sheet of chicken wire composed of a hexagonal network. These sheet polysilicates are classified as phyllosilicates. In these sheets each silicon possesses 1 terminal oxygen and 3 bridging oxygens. The charge density per silicon is -1.
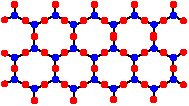
Framework Polysilicates
Framework polysilicates form when each silicon is joined to 4 other silicons via bridging oxygens giving a a 3-dimensional polymeric structure. The monomeric structural unit of these polymers is SiO2. This is an uncharged oxide silica which is no longer basic, but rather acidic. These framework polysilicates are classified as tectosilicates.
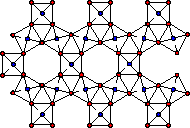

Chemical Weathering
Weathering is a combination of physical and chemical changes which break down rocks and is an important process in the formation of soils.
The basicity of a polysilicate species determines how susceptible that species will be to chemical weathering. The more highly polymerized the polysilicate ions the lower the basicity of those ions. The more basic the polysilicate anion of a mineral, the more readily it will react with weakly acidic solutions causing weathering. Even pure rainwater is slightly acidic due to dissolved CO2. Overtime, rainwater will react with the less polymerized silicate anions, replacing terminal oxygens (lost as water molecules) with bridging oxygens to yield a more highly polymerized silicate. In these processes, the negative charge on the resulting silicate is reduced (negative charge density on each silicon decreased by one for each terminal oxygen lost) allowing cation nutrients in the soil to be washed away. The age and often the fertility of a soil can be determined by the types of polysilicates present.
Soils which contain large amounts of orthosilicates such as olivine are either youthful or present in desert regions. Soils with large amounts of layer silicates such as clay are considered intermediate in age and are often found in terperate regions under a cover of vegitation. These soils are less fertile than the youthful types of soils. Soils with large amounts of acidic silica minerals are often quite infertile because they are incapable of holding onto essential non acidic and feebly acidic nutrient metal ions. In the tropics, soils weather more quickly when exposed due to the hot and rainy climate. Thus, when tropical rain forests are cleared, the soils can maintain nutrient cations to sustain agriculture for a few years.


Western Oregon University
Copyright © 1997 Western Oregon University



