The Physical States and Structures of Oxides
The Gaseous State
In the gaseous state, units (molecules and ion pairs) exert no attractive forces on each other. They remain relatively far apart (the basis of the ideal gas law.) In reality van der Waals forces do exist which induce a temporary dipole in a neighboring molecule. For small units, the van der Waals force between molecules is much weaker than either Coulombic attractions or the forces of covalent bonds. For larger units with numerous highly polarizable electrons, van der Waals forces may be sufficiently strong to hold together molecules at room temperature. Such units would not exist in the gaseous state.| Only monotomic noble gases and small covalent molecules are gases at room temperature. |
The Liquid State
In the liquid state, units are held together by forces that are strong compared to the thermal energy at that temperature. Units are not locked together in extensive, rigid 2- or 3-D lattices. The interunit attractive forces can be stretched so units are able to flow around each other.| Medium-sized covalent molecules or small molecules linked by H-bonds are normally liquids at room temperature. |
The Solid State
Units are packed closely together. The attractive forces between units are sufficiently strong to hold them in position in a lattice despite the vibrational energies of the units.| Large covalent molecules and ionic compound are typically solids at room temperature. |
Large covalent molecules (macromolecules or polymers) often have van der Waals forces which are strong enough to hold the molecules in an extensive, organized framework The melting points of these compounds are usually lower than those of units linked by the much stronger Coulombic forces of ionic salts.
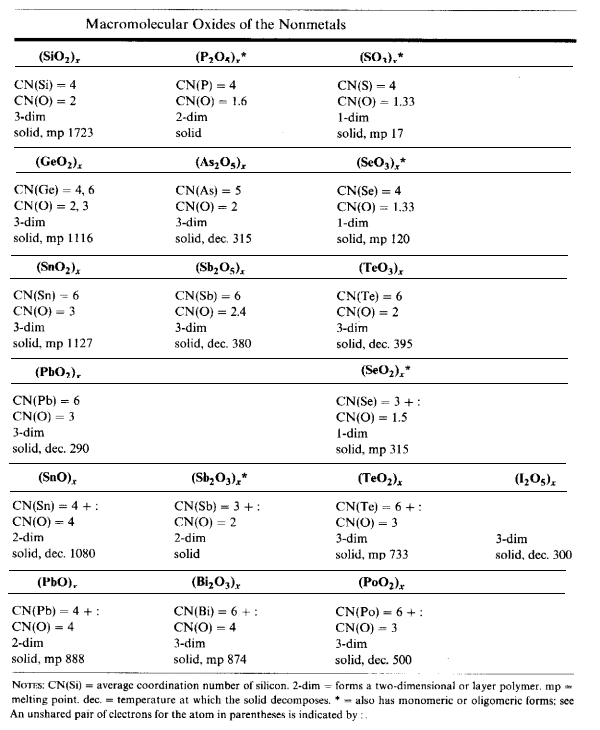
Silicon Dioxide
SiO2 (beta-tridymite form) has units which are linked in a 3-D structure. Each oxygen atom has a CN=2.
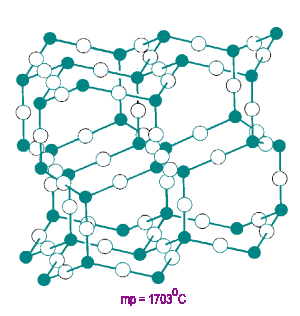
Arsenic (III) Oxide
As2O3 has units linked in a 2-D layered structure. Each oxygen atom has a CN=2.
The unshared pair of p electrons on the arsenic atom block linkage in the third dimension.
Chromium (VI) Oxide
CrO3 is a linear polymer (linked in only one dimension) having an average CN=1.33 for oxygen.
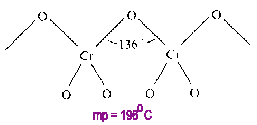
A 1-D polymer has fewer linkages to break and is more flexible than the more highly cross-linked 2- or 3-D polymers on melting. Therefore, the melting point is lower than 2- or 3-D polymers but higher than that of a comparable monomer.
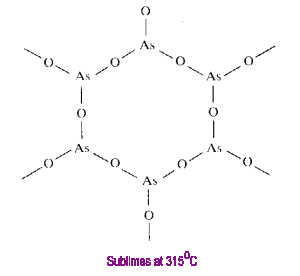
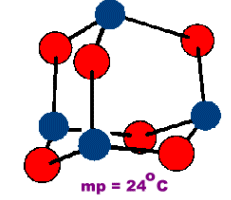
Tetraphosphorus Hexoxide
Oligomeric compounds such as those of the formula M4O6 have a finite molecular size. CN=2 for the oxygens of this structure.
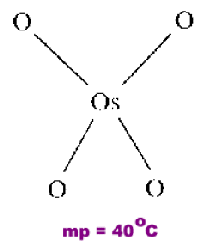
Osmium Tetroxide
OsO4 is an example of a monomeric covalent molecule in which each oxygen has CN=1.
The room temperature physical states, relative melting points and boiling points of compounds are influenced by whether the simplest formula units of the compound are:
|
Periodic Trends in Physical States
Periodic trends in the physical states adopted, the melting points and boiling points of compounds are related to the coordination numbers of the most abundant atoms. Previously, we showed that the coordination numbers of the atoms were related to the ratio of the cationic and anionic radii of the atoms. We can use the radius ratio to predict the coordination number of the atoms and in turn predict the physical state a compound will assume.- Calculate the radius ratio r"cation"/r"anion" and predict the coordination number of the least abundant atom or ion. This will normally be the metal or more electropositive atom.
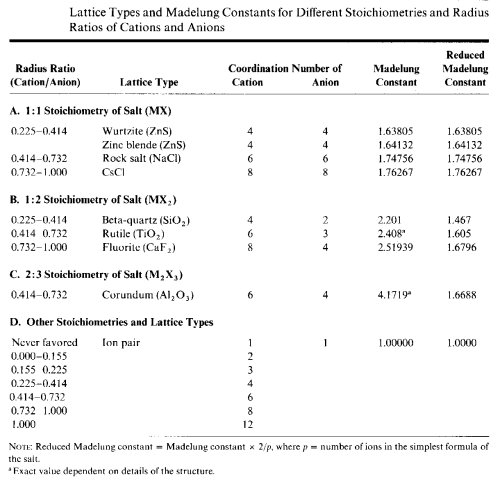
- Calculate the coordination number of the least abundant atom or ion. This atom will usually have the lowest coordination number and may not be able to link into oligomers or polymers.
(CN of metal) x (# M in formula) = (CN of X in formula) x (# X in formula) - If CN=1 for the most abundant atom or ion, the species is predicted to occur as monomeric molecules, with relatively low melting or boiling points. To predict whether it will be a gas, liquid or low-melting solid at room temperature, you wil need to look at the size of the molecule and its H-bonding capability.
- If CN>2 for the most abundant atom or ion, the units will usually link to form polymers having high melting and boiling points. These compounds may decompose into small gaseous molecules before melting or boiling (sublimation).
- For CN between 1 and 2, linkage possibilities may exist to make a 1-D polymer but probably not a more highly cross-linked structure. The closer to one the CN, the greater is the possibility that a small oligomer will be formed.
The table below compares the predicted coordination numbers for the atoms with those observed along with the observed lattice types and melting points of the oxides for the elements of Period 3 and the early elements of Period 6.
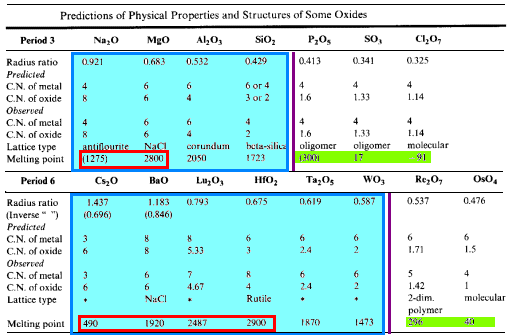
- Solids of high mp are found at the left of the period
- Moving from left to right, the CN of the more abundant atom (oxygen) declines.
When CN drops below 2, the melting point drops sharply- 1-D polymeric, oligomeric and monomeric structures
- Small atoms with high oxidation numbers form the oxides with the lowest mp
- gaseous, liquid or low-melting solid structures
- For metal oxides forming ionic lattices, the melting points and boiling points tend to increase as the charge on the metal increases
- increasing Coulombic forces
 Predict the coordination numbers, physical states, and the relative melting and boiling points of the oxides of Period 2 with the elements in their maximum oxidation state. Predict whether each should be monomeric, oligomeric, polymeric, or in an ionic lattice. For ionic species, try to predict the lattice structure. Predict the coordination numbers, physical states, and the relative melting and boiling points of the oxides of Period 2 with the elements in their maximum oxidation state. Predict whether each should be monomeric, oligomeric, polymeric, or in an ionic lattice. For ionic species, try to predict the lattice structure.
|
Typical trends in melting or boiling points of oxides in their group oxidation states as the oxidation and group numbers increase are shown in the diagram below:
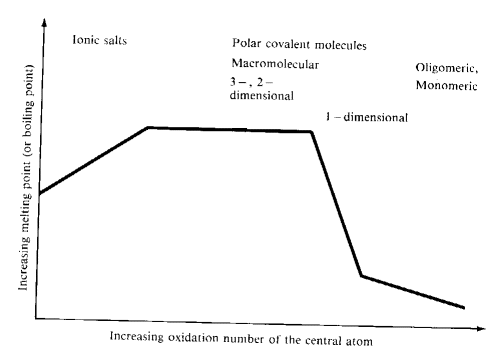
Monomeric molecules are found in the upper right hand portion of the periodic table and also among the d-block elements with oxidation states of +7 and +8. These species possess relatively weak forces between individual units.
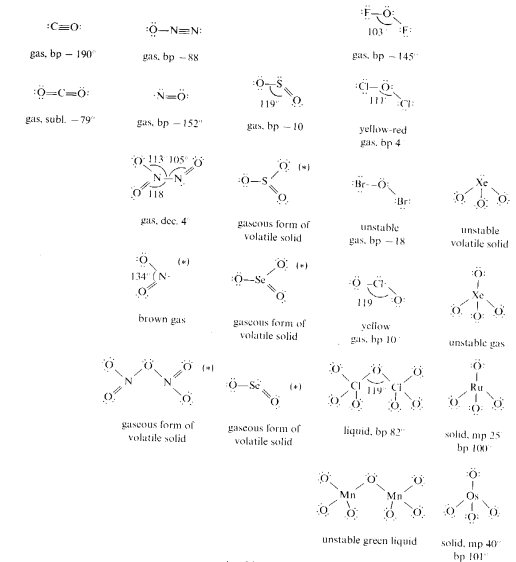
Structures denoted with an asterisk (*) also have alternate oligomeric or polymeric structural forms.
The follow structures are examples of species forming oligomeric molecules.
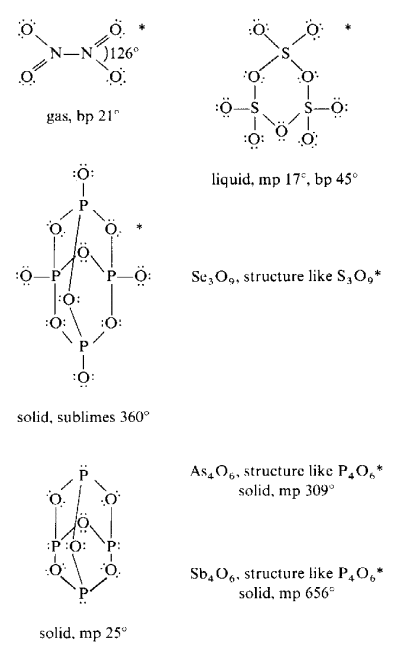
Structures denoted with an asterisk (*) also have alternate oligomeric or polymeric structural forms.
The table below shows important covalent oxides which have polymeric structures.