Redox Chemistry of Nonmetals
Oxidizing Abilities of Nonmetals
When nonmetallic elements act as oxidizing agents, the nonmetal is reduced to its monatomic anion or a protonated form.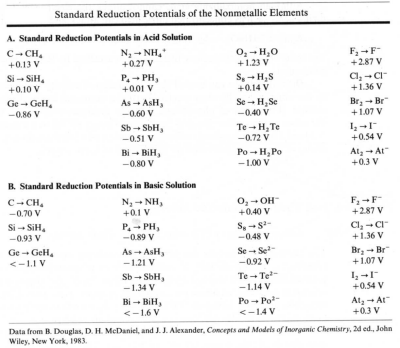
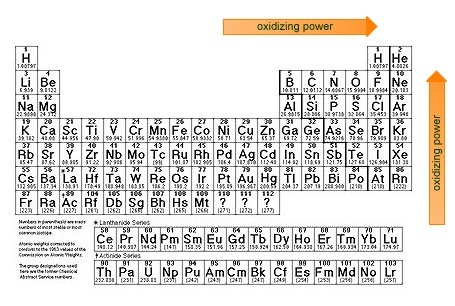
| Oxidizing power increases with increasing electronegativity. |
The monatomic nonmetal anions and their protonated forms usually exhibit the lowest possible oxidation number which gives them the ability to transfer electrons back to other materials (act as reducing agents).
Nonmetals can be divided into two groups based on their redox properties.
- Very electronegative nonmetals
These nonmetals have EN > 2.8 and are good oxidizing agents.
F2 will oxidize all metals and most other substances. It must be stored in either nickel or copper containers because the fluorides of these metals form a film passivating the metal surface.
The other halides are successively less oxidizing.

AlthoughO2 is a strong oxidizing agent, its oxidizing reactions are usually slow kinetically. Many flammable materials will exist in the presence of air until a flame or spark starts the reaction. The very electronegative nonmetals are poor reducing agents. - Electronegative nonmetals These nonmetals have electronegativities between 1.9 and 2.8. These elements have few laboratory uses as oxidizing agents.
Sulfur serves as an oxidizing agent for some electronegative metals. For example, the tarnishing of silver is the formation of silver sulfide.
The electonegative nonmetals have moderate to strong reducing properties.
Basic Behavior of Nonmetal Anions
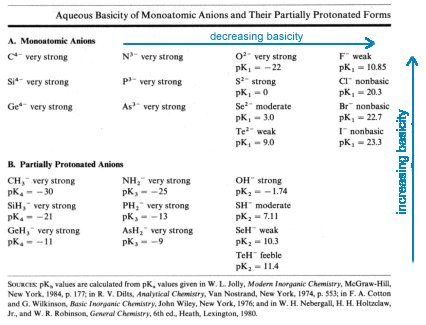
| Basicities increase with increasing negative charge Charges of -3 or -4 can't persist in water |
Periodic Trends in the basicitiy and reducing ability of monoatomic anions:
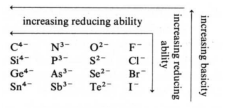
Periodic trends in the acidity of protonation monoatomic anions:

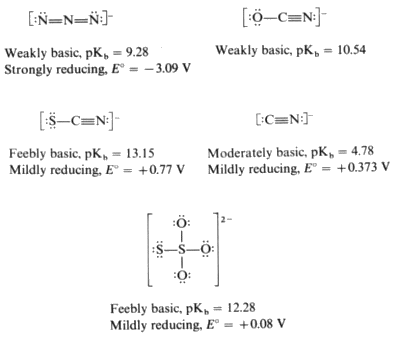
Pseudohalide Ions
Pseudohalide ions are polyatomic anions that resemble halide ions in both their acid-base and redox chemistry. Like the halide ions, they have relatively low basicity generally falling in the feebly to moderately basic categories.
|
The oxidation of the cyanide, thiocyanate and thiosulfate ions generates dimeric species analogously to the oxidation of the halogens.
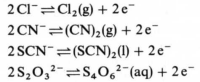
Azide is a powerful reducing agent that will even reduce the sodium ion. Sodium azide produces sodium metal on heating.
Hydride (H-) is both a powerful base and a powerful reducing agent.
Explosives, Flammability and Strong Oxidizing Agents
Compounds which are in very high oxidation states such as oxo anions or oxo acids and elements in very low oxidation states rarely have overlapping predominance areas in Pourbaix diagrams. Therefore they tend to react with each other. These reactions are often extremely exothermic giving off more energy than acid base reactions.Often reactions between species with nonoverlapping predominance areas do not occur immediately because of a high energy of activation. The reaction between strong oxidizing and strong reducing agents often requires an initiator such as a catalyst or source of energy such as a spark. Once initiated, these exothermic reactions may occur so rapidly that a fire or explosion results.
Which of the following appear the most likely to show explosive properties?
|